Vietnamese-made COVID-19 vaccine Nanocovax to go global soon
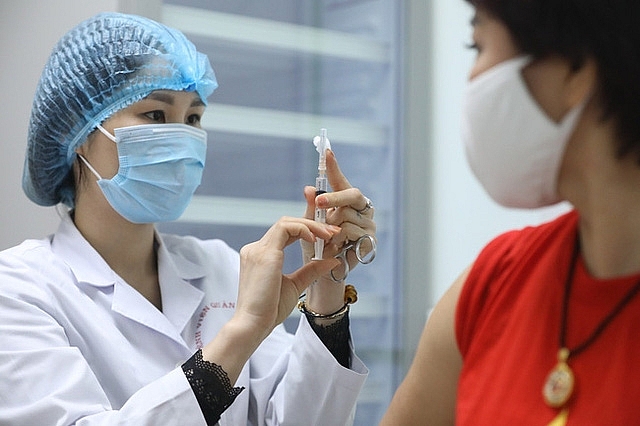 |
| Vietnamese-made COVID-19 vaccine Nanocovax is in the thirrd phase of clinical trials |
Recently, South Korean pharmaceutical company HLB was said to have signed an MoU with Nanogen Pharmaceutical Biotechnology JSC to acquire the rights to supply the Vietnamese COVID-19 vaccine Nanocovax across the globe, except for Vietnam and India.
The two sides agreed with the transfer of technology for the Korean firm to produce and sell the Vietnamese partner's vaccine, as well as to carry out marketing campaigns globally. They plan to finish negotiations once scientists finish reviewing the clinical data on Nanocovax within the next three months.
Nanocovax is Vietnam's domestically developed coronavirus vaccine candidate. The vaccine is in the phase of clinical trials.
In early August, India’s Vekaria Pharmaceutical Group inked a deal with Nanogen to supply Nanocovax to the world's second-most populous country.
The deals are expected to help the Vietnamese-made COVID-19 vaccine soon join global supply chain, easing the global shortfall of COVID-19 vaccines.
 Nanogen ties up with Vekaria Healthcare LLP to produce Nanocovax vaccine Nanogen ties up with Vekaria Healthcare LLP to produce Nanocovax vaccine |
 Volunteers receive second shots of Nanocovax vaccine Volunteers receive second shots of Nanocovax vaccine |
 Volunteers get 2nd shot of 25mcg dose of Nanocovax Volunteers get 2nd shot of 25mcg dose of Nanocovax |
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Themes: Healthcare Platform
- PM outlines new tasks for healthcare sector
- Opella and Long Chau join forces to enhance digestive and bone health
- Hanoi intensifies airport monitoring amid Nipah disease risks
- Cosmetics rules set for overhaul under draft decree
- Policy obstacles being addressed in drug licensing and renewal
Related Contents
Latest News
More News
- VIFC in Ho Chi Minh City officially launches (February 12, 2026 | 09:00)
- VNPAY and NAPAS deepen cooperation on digital payments (February 11, 2026 | 18:21)
- Vietnam financial markets on the rise amid tailwinds (February 11, 2026 | 11:41)
- New tax incentives to benefit startups and SMEs (February 09, 2026 | 17:27)
- VIFC launches aviation finance hub to tap regional market growth (February 06, 2026 | 13:27)
- Vietnam records solid FDI performance in January (February 05, 2026 | 17:11)
- Manufacturing growth remains solid in early 2026 (February 02, 2026 | 15:28)
- EU and Vietnam elevate relations to a comprehensive strategic partnership (January 29, 2026 | 15:22)
- Vietnam to lead trade growth in ASEAN (January 29, 2026 | 15:08)
- Japanese business outlook in Vietnam turns more optimistic (January 28, 2026 | 09:54)

 Tag:
Tag:
























 Mobile Version
Mobile Version