Vietnam aims to successfully produce at least one locally-made COVID-19 vaccine in 2021
 |
| Deputy Minister of Health Tran Van Thuan chaired the meeting on July 17 with a plan to successfully produce at least one locally-made COVID-19 vaccine in 2021 |
At the virtual meeting of the special working group on vaccine research and clinical trial, and the development of COVID-19 vaccines, participants discussed plansto invite more experts from the World Health Organization (WHO) to support COVID-19 vaccine development in Vietnam.
Additionally, they discussed the acceleration of clinical trials for the two locally-made COVID-19 vaccines Nano Covax, and COVIVAC, as well as the transfer of technology for COVID-19 vaccine production, among others.
The prime minister invitedWHO experts to support Vietnam in research and production of the vaccine so that Vietnam can accelerate locally-made vaccine production to ensure itself vaccine supply.
The deputy minister of health assigned relevant departments and agencies of the Ministry of Health (MoH) to maximally support units in the research, production, and import of vaccines, and license vaccines from emergency use.
The MoH plans to soon submit to the government plans to use the national COVID-19 vaccine fund to support the third phase of clinical trials for locally-made vaccines while proposing the government to direct relevant ministries, agencies, and localities to work with the MoH on clinical trials in cities and provinces to ensure safety and efficacy while remaining on schedule.
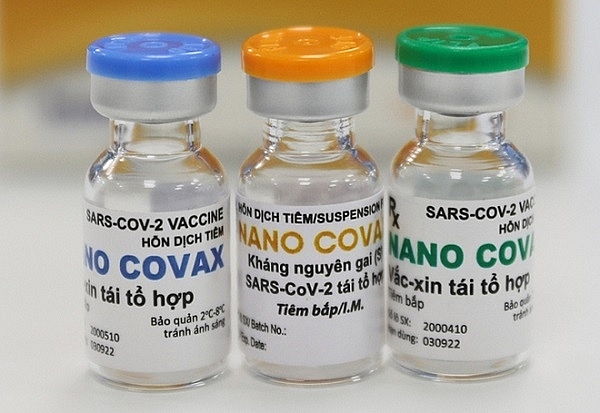 |
| The locally-made Nano Covax vaccine |
Earlier on July 14, to fast-track the clinical trial and development of locally-made COVID-19 vaccines in line with the prime minister’s directions, Minister of Health Nguyen Thanh Long signed Decision No.3439/QD-BYT on establishing a special working group on research and development of COVID-19 vaccines led by Deputy Minister Tran Van Thuan.
At present, Nano Covax developed by the Nanogen Pharmaceutical Biotechnology JSC is in the third phase of clinical trials. From June to mid-July, over 14,000 volunteers participating in the third clinical trial got their first shots with positive results.
As planned, the second shot of Nano Covax will be administered before August 15. If the third clinical trial produces good results and is accepted by the National Ethics Council, the vaccine will be considered for approval for emergency use.
According to the producer, it can roll out 5-10 million doses a month. In addition to Nano Covax, COVIVAC developed by the Institute of Vaccines and Medical Biologicals (IVAC) is in the second phase of clinical trial.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
 Tag:
Tag:
Themes: Healthcare Platform
- Opella and Long Chau join forces to enhance digestive and bone health
- Hanoi intensifies airport monitoring amid Nipah disease risks
- Cosmetics rules set for overhaul under draft decree
- Policy obstacles being addressed in drug licensing and renewal
- Sanofi, Long Chau Pharmacy relaunch medicine blister pack collection initiative
Related Contents
Latest News
More News
- Hanoi intensifies airport monitoring amid Nipah disease risks (January 29, 2026 | 15:21)
- Congratulations from VFF Central Committee's int’l partners to 14th National Party Congress (January 25, 2026 | 09:46)
- List of newly-elected members of 14th Political Bureau announced (January 23, 2026 | 16:27)
- 14th Party Central Committee unanimously elects To Lam as General Secretary (January 23, 2026 | 16:22)
- List of members of 14th Party Central Committee announced (January 23, 2026 | 09:12)
- Highlights of fourth working day of 14th National Party Congress (January 23, 2026 | 09:06)
- Press provides timely, accurate coverage of 14th National Party Congress (January 22, 2026 | 09:49)
- Press release on second working day of 14th National Party Congress (January 22, 2026 | 09:19)
- Minister sets out key directions to promote intrinsic strength of Vietnamese culture (January 22, 2026 | 09:16)
- 14th National Party Congress: Renewed momentum for OVs to contribute to homeland (January 21, 2026 | 09:49)





























 Mobile Version
Mobile Version