Vietnam approves sixth COVID-19 vaccine for emergency use
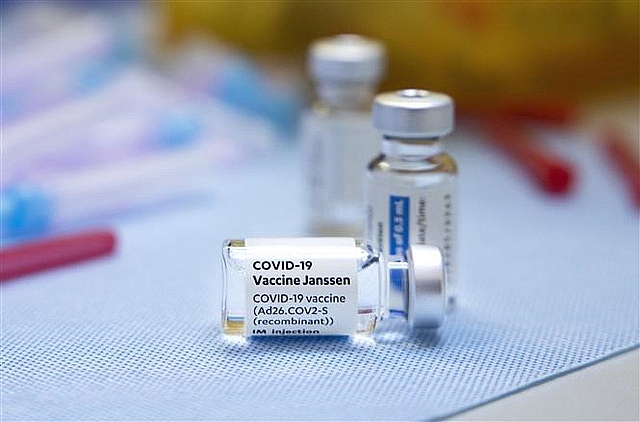 |
| The COVID-19 Vaccine Janssen |
The vaccine is produced by Janssen Pharmaceutica NV (Belgium) and Janssen Biologics BV (the Netherlands).
This is the sixth kind of COVID-19 vaccine that Vietnam has approved with conditions for emergency use. The five others are Comirnaty of Pfizer, A2D1222 of AstraZeneca, Sputnik-V of Gamalaya, Vero-Cell of Sinopharm, and Spikevax of Moderna.
The move helps the country increase the vaccine supply amid worst outbreak in the southern region so far.
The MoH has assigned the Drug Administration of Vietnam (DAV) to grant the import licence for the vaccine when it receives the documents of authorised importers in line with the prevailing rules.
The ministry also asked the Agency of Science, Technology and Training to select qualified units to assess the efficacy and safety of the vaccine.
Meanwhile, the National Institute of Vaccine and Medical Biology will need to verify the factory certificate of Janssen vaccines.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Themes: Healthcare Platform
- Hanoi intensifies airport monitoring amid Nipah disease risks
- Cosmetics rules set for overhaul under draft decree
- Policy obstacles being addressed in drug licensing and renewal
- Sanofi, Long Chau Pharmacy relaunch medicine blister pack collection initiative
- Takeda Vietnam awarded for ongoing support of Vietnam’s sustainability efforts
Related Contents
Latest News
More News
- Hanoi intensifies airport monitoring amid Nipah disease risks (January 29, 2026 | 15:21)
- List of newly-elected members of 14th Political Bureau announced (January 23, 2026 | 16:27)
- 14th Party Central Committee unanimously elects To Lam as General Secretary (January 23, 2026 | 16:22)
- List of members of 14th Party Central Committee announced (January 23, 2026 | 09:12)
- Highlights of fourth working day of 14th National Party Congress (January 23, 2026 | 09:06)
- Press provides timely, accurate coverage of 14th National Party Congress (January 22, 2026 | 09:49)
- Press release on second working day of 14th National Party Congress (January 22, 2026 | 09:19)
- Minister sets out key directions to promote intrinsic strength of Vietnamese culture (January 22, 2026 | 09:16)
- 14th National Party Congress: Renewed momentum for OVs to contribute to homeland (January 21, 2026 | 09:49)
- Party Congress building momentum for a new era of national growth (January 20, 2026 | 15:00)



 Tag:
Tag:

























 Mobile Version
Mobile Version