Open doors for pandemic-related pharma imports
The ministry (MoH) on May 6 issued Document No.3740/BYT-TB-CT on a list of biological products and medical equipment for in-vitro diagnostic tests for coronavirus that are to receive registration numbers and licences for imports, thus enabling health departments, hospitals, and research institutes to take the initiative in purchasing these for prevention and use.
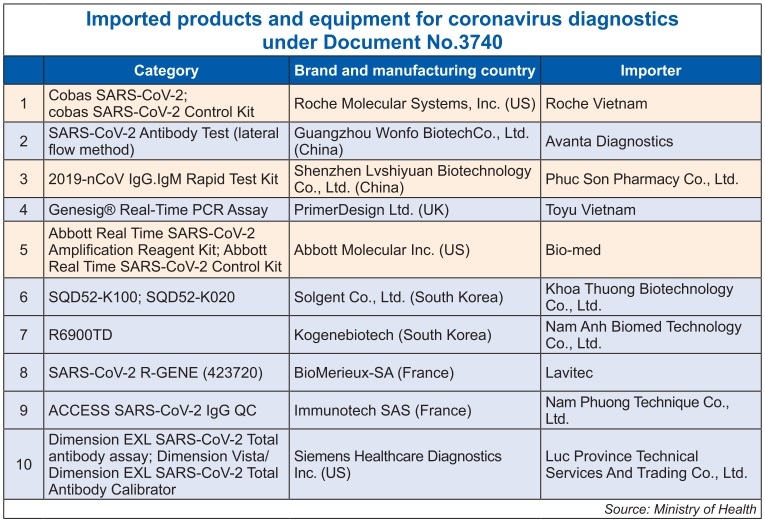 |
The list of 26 domestic and international suppliers features prominent names such as Roche Diagnostics, Abbott, Siemens Healthcare Diagnostics, and more.
The move was made following direction from the Secretariat of the Party Central Committee, the government, and head of the National Steering Committee for COVID-19 Prevention and Control as a way to decentralise and encourage units in taking initiative and utilising flexibility in prevention and fight against the global health crisis.
The list will create more opportunities for foreign manufacturers and suppliers to increase their coverage of products in Vietnam, which is experiencing its latest batch of cases in several provinces.
Nguyen Van Dung from BIO-MED JSC – the importer of Abbott Real Time SARS-CoV-2 amplification Reagent Kit and a control kit produced by Abbott Molecular Inc. – told VIR last week, “The imports serve the pandemic prevention and fight in several hospitals, and we are focusing more on these kinds of products to meet local demand. This week we expect to install kits at Tu Du Hospital and Ho Chi Minh City Hospital for Tropical Diseases, and some more are coming.”
So far, the products are installed at some leading hospitals in the north like Bach Mai Hospital and 108 Military Central Hospital; and some in the south, including Cho Ray Hospital.
Similarly, Siemens Healthcare Diagnostics Inc., Roche Diagnostics GmbH, and Roche Molecular Systems Inc. have some products on the list (see box). Other famous names include DiaSorin S.p.A. from Italy, Seegene Inc. from South Korea, Japan’s Fujirebio Inc., and British-based PrimerDesign Ltd.
International biology products, as well as medical equipment manufacturers and providers, have made success in Vietnam. The country is striving towards achieving universal health coverage, looking to expand its private hospital sector, and exploring new ways to improve quality and access to healthcare, such as with the use of digital platforms.
Siemens Healthcare Diagnostics has been in Vietnam for decades. With an innovative portfolio of performance-driven solutions covering major segments including clinical chemistry, hemostasis, and molecular diagnostic and point-of-care testing – combined to streamline workflow and support improved patient outcomes – Siemens has been contributing to the development of the Vietnamese healthcare sector.
Elsewhere, Roche Diagnostics Vietnam has been present in the country since 2001. It accesses healthcare and diagnostics via an approach focusing on disease awareness, screening and early diagnosis, healthcare capacity, and sustainable funding solutions.
The local medical device and diagnostics market reports double-digit annual growth, with 90 per cent made up of imports. To meet growing demands, Vietnam’s import of medical devices and diagnostics has been rising annually, and it is forecast that the industry will become even more bustling as more multinational corporations expand to and within the market.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Themes: Healthcare Platform
- PM outlines new tasks for healthcare sector
- Opella and Long Chau join forces to enhance digestive and bone health
- Hanoi intensifies airport monitoring amid Nipah disease risks
- Cosmetics rules set for overhaul under draft decree
- Policy obstacles being addressed in drug licensing and renewal
Related Contents
Latest News
More News
- VNPAY and NAPAS deepen cooperation on digital payments (February 11, 2026 | 18:21)
- Vietnam financial markets on the rise amid tailwinds (February 11, 2026 | 11:41)
- New tax incentives to benefit startups and SMEs (February 09, 2026 | 17:27)
- VIFC launches aviation finance hub to tap regional market growth (February 06, 2026 | 13:27)
- Vietnam records solid FDI performance in January (February 05, 2026 | 17:11)
- Manufacturing growth remains solid in early 2026 (February 02, 2026 | 15:28)
- EU and Vietnam elevate relations to a comprehensive strategic partnership (January 29, 2026 | 15:22)
- Vietnam to lead trade growth in ASEAN (January 29, 2026 | 15:08)
- Japanese business outlook in Vietnam turns more optimistic (January 28, 2026 | 09:54)
- Foreign leaders extend congratulations to Party General Secretary To Lam (January 25, 2026 | 10:01)

 Tag:
Tag:




















 Mobile Version
Mobile Version