Questions arise over diabetes drug recall
 |
“Worried and scared” were the first emotions mentioned by Mai Nguyen, a 54-year-old woman living in the Linh Dam residential area in Hanoi, when she heard that a batch of metformin tablets had been recalled in the United States because they contain high levels of cancer-causing contaminant N-nitrosodimethylamine (NDMA) – and Vietnamese authorities were following suit with enquiries into batches in this country.
The drugs, popularly used to treat type 2 diabetes, have been distributed to wholesalers worldwide.
“It is so terrible. I have been living with type 2 diabetes for seven years so I often use Glucophage 850 mg. I do not know about NDMA, but I have to use the drug,” Nguyen told VIR. “Who will be responsible if Glucophage, or other metformin drugs, are found with a higher-than-accepted intake limit,” she asked.
“Many of my friends with the disease are also worried,” she added. “We expect a list of cancer-causing metformin drugs imported into Vietnam to be published soon so that we can take precautions.”
Following warnings from international health agencies, the Drug Administration of Vietnam (DAV) under the Ministry of Health in a document asked manufacturers and importers of metformin-contained pharmaceuticals to take action to ensure quality and safety to users.
Manufacturers are being asked to ensure the quality of the drug and ensure the amount of NDMA does not surpass the acceptable limit – not higher than 0.32 parts per million as ruled by global ICH standards on assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk.
Manufacturers are also requested to assess metformin-contained material suppliers, including the origins and quality, while checking the production process to ensure that NDMA cannot rise during production, preservation, or distribution.
For importers, the DAV asked them to strictly assess material suppliers and manufacturers, and only import the materials and drugs ensuring metformin quality standards and those on NDMA.
The DAV also ordered the National Institute of Drug Quality Control and the Ho Chi Minh City equivalent to work with related institutes of other cities and provinces on analysis of metformin drugs, while taking samples to test the quality of materials and pharmaceuticals and then report to the DAV to make the next steps.
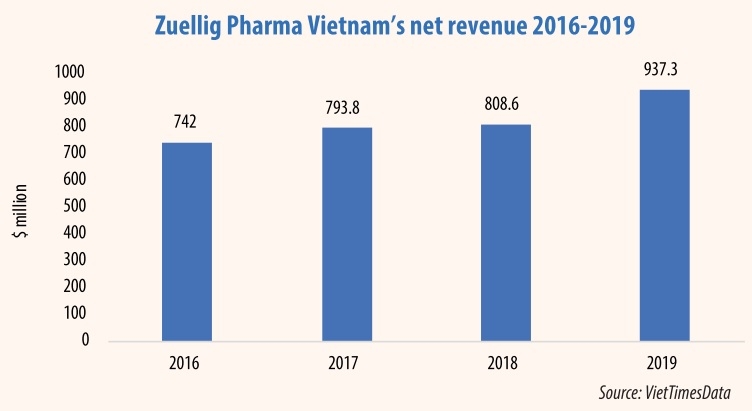
Products containing metformin are being sold at drugstores, with Glucophage 500mg or 850mg film-coated tablets being among the most popular. Glucophage is manufactured in France by Merck Sante S.A.S and imported by Zuellig Pharma Vietnam.
“Glucophage is being the best seller at our drugstore. Most people with diabetes come to buy this drug because they are prescribed,” said a pharmacist at a Linh Dam drugstore in Hanoi.
Zuellig Pharma Vietnam was asked for comment by VIR on the issue but has not yet been forthcoming.
Other popular versions include Glucofine 500mg or 850mg manufactured by Domesco, and Metformin Stada manufactured by Stada Vietnam in the southern province of Binh Duong.
While Vietnamese institutes have not announced results of testing yet, questions are once again being raised about pharmaceutical firms’ ethics, with groups accused of putting profit before people’s health.
Previously, the Health Sciences Authority of Singapore, Health Canada, the US Food & Drug Administration (FDA), and others found that metformin drugs containing NDMA above the acceptable intake limit can cause risk of cancer.
Last year, the FDA recommended five pharmaceutical companies to voluntarily recall metformin after the agency found high levels of a possible cancer-causing impurity in some versions of the medication. In January, the FDA announced that the recall extends to additional manufacturers, forms, and dosages.
Thus far, 11 companies have voluntarily withdrawn 500mg, 750mg, and 1000mg extended-release metformin tablets and extended-release metformin liquid formulation.
| Pharma firms in voluntary recall of metformin drugs Amneal Pharmaceuticals Apotex Corp. AVKARE Inc. (Amneal) Bayshore Pharmaceuticals, LLC Denton Pharma, Inc. (Marksans) Direct Rx (Marksans) Granules Pharmaceuticals Lupin Pharmaceuticals Marksans Pharma Ltd. Nostrum Laboratories, Inc. PD-Rx Pharmaceuticals (Amneal) PD-Rx Pharmaceuticals (Marksans) Preferred Pharmaceuticals, Inc. (Marksans) RemedyRepack Inc. (Marksans) Sun Pharmaceuticals Industries, Inc. Teva Pharmaceuticals |
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Themes: Healthcare Platform
- Hanoi intensifies airport monitoring amid Nipah disease risks
- Cosmetics rules set for overhaul under draft decree
- Policy obstacles being addressed in drug licensing and renewal
- Sanofi, Long Chau Pharmacy relaunch medicine blister pack collection initiative
- Takeda Vietnam awarded for ongoing support of Vietnam’s sustainability efforts
Latest News
More News
- Foreign leaders extend congratulations to Party General Secretary To Lam (January 25, 2026 | 10:01)
- 14th National Party Congress wraps up with success (January 25, 2026 | 09:49)
- Congratulations from VFF Central Committee's int’l partners to 14th National Party Congress (January 25, 2026 | 09:46)
- 14th Party Central Committee unanimously elects To Lam as General Secretary (January 23, 2026 | 16:22)
- Worldwide congratulations underscore confidence in Vietnam’s 14th Party Congress (January 23, 2026 | 09:02)
- Political parties, organisations, int’l friends send congratulations to 14th National Party Congress (January 22, 2026 | 09:33)
- Press release on second working day of 14th National Party Congress (January 22, 2026 | 09:19)
- 14th National Party Congress: Japanese media highlight Vietnam’s growth targets (January 21, 2026 | 09:46)
- 14th National Party Congress: Driving force for Vietnam to continue renewal, innovation, breakthroughs (January 21, 2026 | 09:42)
- Vietnam remains spiritual support for progressive forces: Colombian party leader (January 21, 2026 | 08:00)

 Tag:
Tag:














 Mobile Version
Mobile Version