Vietnam approves eighth COVID-19 vaccine for emergency use
Vietnam’s Ministry of Health on September 17 issued Decision No.4471/QD-BYT approving the Cuban COVID-19 vaccine Abdala for emergency use.
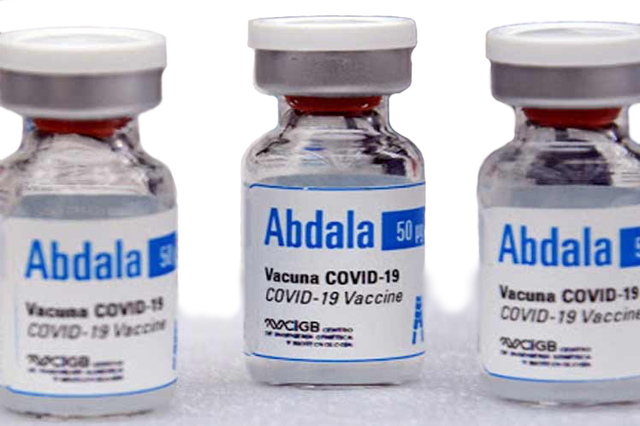 |
| Abdala is the eight vaccine approved for emergency use |
COVID-19 vaccine Abdala is produced by AICA Laboraries, Base Business Unit (BBU) AICA – Cuba.
To date, Vietnam has approved eight COVID-19 vaccines for emergency use. They are AstraZeneca, Sputnik V, COVID-19 Vaccine Janssen (Johnson & Johnson), Spikevax (COVID-19 Vaccine Moderna), Comirnaty (Pfizer – BioNTech), Vero Cell (China National Biotec Group (CNBG)/ Sinopharm) Hayat - Vax and Abdala.
Vietnam has so far received more than 38 million doses of COVID-19 vaccines, and vaccinates over 33 million doses.
 India and Vietnam team up in vaccine collaboration India and Vietnam team up in vaccine collaboration |
 Vietnam receives nearly 1.5 million doses of COVID-19 vaccines from France and Italy Vietnam receives nearly 1.5 million doses of COVID-19 vaccines from France and Italy |
 Hanoi reports 22 new COVID-19 cases on September 13 morning Hanoi reports 22 new COVID-19 cases on September 13 morning |
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Themes: Healthcare Platform
- PM outlines new tasks for healthcare sector
- Opella and Long Chau join forces to enhance digestive and bone health
- Hanoi intensifies airport monitoring amid Nipah disease risks
- Cosmetics rules set for overhaul under draft decree
- Policy obstacles being addressed in drug licensing and renewal
Related Contents
Latest News
More News
- US-Iran conflict affecting asset prices (March 04, 2026 | 09:17)
- Middle East tensions set to test VN-Index, boost energy stocks (March 02, 2026 | 17:15)
- Agency of Foreign Trade warns of trade disruption due to Middle East conflict (March 02, 2026 | 17:11)
- Spring Fair 2026 boosts domestic demand (March 02, 2026 | 16:30)
- Law on Investment takes effect (March 02, 2026 | 16:21)
- Ho Chi Minh City attracts nearly $980 million in FDI in early 2026 (March 02, 2026 | 10:57)
- Academic-policy network planned to support VIFC development (February 28, 2026 | 08:00)
- Businesses bouncing back after turbulent year (February 27, 2026 | 16:42)
- VinaCapital launches Vietnam's first two strategic-beta ETFs (February 26, 2026 | 09:00)
- PM sets five key tasks to accelerate sci-tech development (February 26, 2026 | 08:00)

 Tag:
Tag:























 Mobile Version
Mobile Version