Pharma rules unable to stand test of time
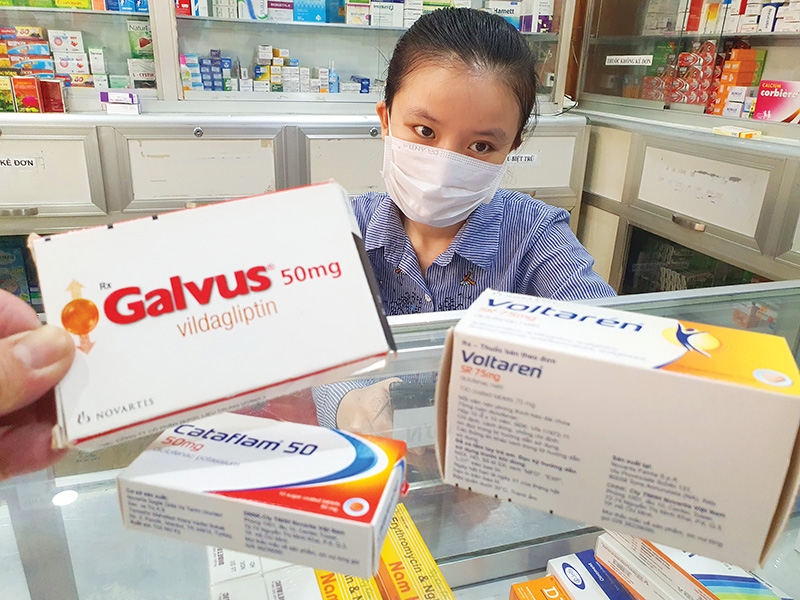 |
| Foreign pharmaceuticals are pushing for a reform of import rules. Photo: Le Toan |
Decree No.54/2017/ND-CP which entered into force in July 2017 to guide the implementation of the Law on Pharmacy was expected to be a breath of fresh air in the pharmaceuticals market. However, troubles still linger in regards to lengthy procedures and drug registration, with the long-awaited distribution rights remaining far from the grasp of Adamed Pharma and other foreign-invested enterprises (FIEs).
According to Magdalena Krakowiak, head of Public Affairs and CSR in Vietnam at Adamed Pharma, the changes in 2017 were supposed to be a step towards granting FIEs the right to directly import pharmaceutical products and sell them to Vietnam-based wholesalers. However, FIEs are still not allowed to distribute pharmaceutical products.
“The changes from Decree 54 were not that meaningful from the perspective of Adamed. From the beginning, the company has been eyeing up the incentives from the country’s plan to prioritise domestically manufactured pharmaceuticals,” she told VIR.
| Magdalena Krakowiak - Head of Public Affairs & CSR in Vietnam, Adamed Pharma
When in 2017 Adamed acquired a controlling stake in Davipharm, one of the fastest-growing pharmaceutical companies in Vietnam, we were quite clear that we wanted to expand our local production. With over $10 million invested by Adamed by the end of 2020 in Davipharm’s production plant in Binh Duong province, Vietnam’s first high-potency zone for production of oncological drugs in solid forms and with EU-GMP certification, we continue to execute our strategy focused on raising standards of drug production in Vietnam. The company’s ambition is to offer domestically produced and high-quality medicines at affordable prices to Vietnamese patients. By establishing the local production of these affordable drugs, the company plans to expand to other markets, with Vietnam as a production hub. Davipharm’s portfolio has 28 high potency drugs, including oncology drugs for the treatment of various cancers and leukaemia. In general, Davipharm provides medicines for 12 different therapeutic areas including the cardiovascular and respiratory systems. Through its local company Davipharm, Adamed aims to increase the capacity of domestic drug manufacturers, improve patient safety, and provide medicines to treat some of the most common diseases in Vietnam. |
Krakowiak added that the additional administrative burdens resulting from the Ministry of Health’s (MoH) Circular No.32/2018/TT-BYT dated November 12, 2018 on marketing authorisation of drugs and medicinal ingredients such as drug registration requirements, which are not in line with international standards and created lengthy procedures, forced new barriers for importers.
Like Adamed, other FIEs have been facing similar challenges.
“In the four years since the issuance of Decree 54, we our members have been finding it hard to expand our business activities in Vietnam because of the ban on the distribution of pharmaceuticals among FIEs,” confirmed a representative of an international pharma firm, who denied to be named.
Specifically, FIEs claim processing applications to renew marketing authorisation runs far beyond the set timeline, and new submissions are not processed. To boot, the provisions of Circular No.29/2020/TT-BYT issued at the end of 2020 will soon elapse, bringing the situation back to the regulations of Circular 32.
Over the past years, to adapt to the new rules and to benefit from the right to directly import pharmaceutical products, multinational corporations have been changing their business strategies. France’s Sanofi-Aventis Vietnam Ltd. has turned it into the first lawful multinational importer in drug production in the country since 2019.
A representative of Sanofi Vietnam said, “Sanofi Vietnam always desires to bring more and more innovative healthcare solutions to Vietnam which can help people enjoy a healthier and fuller life. We always respect the regulations and instructions of local authorities, and we also work closely with the MoH, its Drug Administration of Vietnam, and other health authorities as well as follow their instructions.”
Switzerland-based Novartis has also inaugurated a new legal entity in Vietnam since early 2020, when it became one of the first MNC in the country to successfully transform from a representative office to a foreign importer. Elsewhere, AstraZeneca transformed its Vietnamese arm into an FIE by launching AstraZeneca Vietnam last year. With the transition, MNCs are now increasing their role in Vietnam’s pharmaceutical market, where 50 per cent of pharmaceuticals are imported.
Statistics from Vietnam Customs show that Vietnam spent nearly $1.2 billion on pharmaceutical imports in the first five months of 2021, down 5.8 per cent on-year. The main markets are France, Germany, the United States, India, Italy, the United Kingdom, and Belgium.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Themes: Healthcare Platform
- PM outlines new tasks for healthcare sector
- Opella and Long Chau join forces to enhance digestive and bone health
- Hanoi intensifies airport monitoring amid Nipah disease risks
- Cosmetics rules set for overhaul under draft decree
- Policy obstacles being addressed in drug licensing and renewal
Related Contents
Latest News
More News
- PM outlines new tasks for healthcare sector (February 25, 2026 | 16:00)
- Masan Consumer names new deputy CEO to drive foods and beverages growth (February 23, 2026 | 20:52)
- Myriad risks ahead, but ones Vietnam can confront (February 20, 2026 | 15:02)
- Vietnam making the leap into AI and semiconductors (February 20, 2026 | 09:37)
- Funding must be activated for semiconductor success (February 20, 2026 | 09:20)
- Resilience as new benchmark for smarter infrastructure (February 19, 2026 | 20:35)
- A golden time to shine within ASEAN (February 19, 2026 | 20:22)
- Vietnam’s pivotal year for advancing sustainability (February 19, 2026 | 08:44)
- Strengthening the core role of industry and trade (February 19, 2026 | 08:35)
- Future orientations for healthcare improvements (February 19, 2026 | 08:29)


 Tag:
Tag:




















 Mobile Version
Mobile Version