Vietnam approves Cuba’s Abdala vaccine with conditions
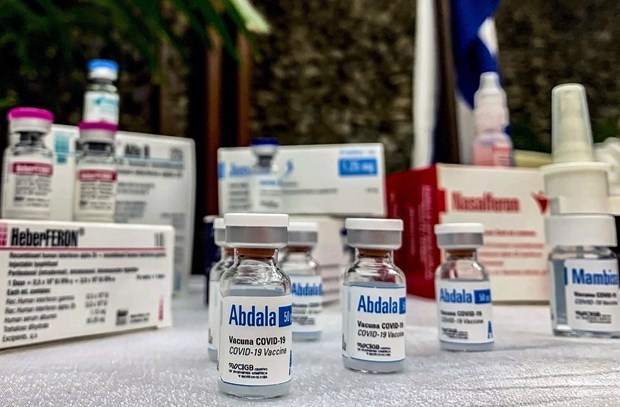 |
| The Ministry of Health approves with conditions for the emergency use of Cuba's Abdala. (Photo: AFP/VNA) |
Hanoi - The Ministry of Health (MoH) on September 17 approved with conditions for the emergency use of Cuba's Abdala COVID-19 vaccine in COVID-19 prevention and control.
The vaccine by manufactured at the AICA Laboratories Company and Base Business Unit (BBU) AICA in Cuba.
The Centre for Immunisation Vaccines Polyvac in Vietnam asked for permission for the vaccine to be used.
The MoH insisted on a number of conditions before granting approval.
It has stated that the Drug Administration of Vietnam is responsible for licensing Abdala vaccine based on regulations for importation and quality management.
The Department of Science, Technology and Training has been given responsibility for selecting a unit that is qualified to evaluate the vaccine’s safety and effectiveness.
The General Department of Preventive Medicine is in charge of injecting the vaccine.
And finally, the National Institute for Control of Vaccine and Biologicals is responsible for verifying and issuing certificates for the vaccine before it can be used.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Related Contents
Latest News
More News
- Election committees set voting hours for polls (March 09, 2026 | 13:25)
- Vietnamese NA helps build institutional foundations for long-term development: Canadian researcher (March 09, 2026 | 12:39)
- NA Vice Chairman inspects election preparations in southernmost Ca Mau province (March 09, 2026 | 12:36)
- Hanoi police roll out comprehensive security for upcoming elections (March 07, 2026 | 10:00)
- Finance Minister Nguyen Van Thang meets voters in Dien Bien ahead of NA elections (March 06, 2026 | 17:23)
- NA chairman urges thorough preparations ahead of March 15 elections (March 06, 2026 | 17:21)
- Hanoi thoroughly prepares for NA, People’s Council election (March 06, 2026 | 14:13)
- Early voting completed in Con Dao special zone (March 06, 2026 | 14:11)
- K’Ho ethnic minority group in Lam Dong province looks forward to Election Day (March 06, 2026 | 14:07)
- Voters proud to contribute directly to national milestone (March 06, 2026 | 14:03)

 Tag:
Tag:




















 Mobile Version
Mobile Version