Japan approves Ebola drug remdesivir for COVID-19 treatment
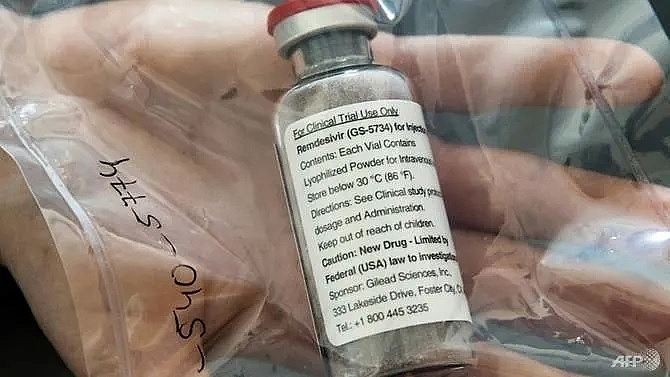 |
| Remdesivir should get the green light in Japan after already receiving approval in the US AFP/Ulrich Perrey |
This makes Japan the second country to approve the drug after US regulators authorised it on Friday for emergency use against severe cases of COVID-19.
"Remdesivir was approved under exceptional measures," a health, labour and welfare ministry official said.
"It was our country's first such approval for the treatment of coronavirus patients," the official told AFP.
Prime Minister Shinzo Abe said last week the government was getting ready to give a speedy green light to the experimental drug developed by US firm Gilead Sciences.
The US go-ahead came after a major clinical trial showed remdesivir - originally developed to treat Ebola - shortened the time to recovery in some patients by a third. The difference in mortality rate was not statistically significant.
Remdesivir, which is administered by injection, was already available to some patients who enrolled in clinical trials around the world.
As for Avigan, developed by Japanese firm Fujifilm Toyama Chemical, Suga said the government "aims to approve it this month" if a clinical trial involving 100 patients proves effective.
The drug, whose generic name is favipiravir, was approved for use in Japan in 2014 but only in flu outbreaks that are not being effectively addressed by existing medications.
It is not available on the market and can only be manufactured and distributed at the request of the Japanese government.
Favipiravir, which can be taken orally as a pill, works by blocking the ability of a virus to replicate inside a cell.
Remdesivir incorporates itself into the virus's genome, short-circuiting its replication process.
Avigan has been shown in animal studies to affect foetal development, meaning it is not given to pregnant women.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
 Tag:
Tag:
Themes: COVID-19
- 67 million children missed out on vaccines because of Covid: UNICEF
- Vietnam records 305 COVID-19 cases on October 30
- 671 new COVID-19 cases recorded on October 1
- Vietnam logs additional 2,287 COVID-19 cases on Sept. 21
- People’s support decisive to vaccination coverage expansion: official
Related Contents
Latest News
More News
- Foreign leaders extend congratulations to Party General Secretary To Lam (January 25, 2026 | 10:01)
- Russian President congratulates Vietnamese Party leader during phone talks (January 25, 2026 | 09:58)
- Worldwide congratulations underscore confidence in Vietnam’s 14th Party Congress (January 23, 2026 | 09:02)
- Political parties, organisations, int’l friends send congratulations to 14th National Party Congress (January 22, 2026 | 09:33)
- 14th National Party Congress: Japanese media highlight Vietnam’s growth targets (January 21, 2026 | 09:46)
- 14th National Party Congress: Driving force for Vietnam to continue renewal, innovation, breakthroughs (January 21, 2026 | 09:42)
- Vietnam remains spiritual support for progressive forces: Colombian party leader (January 21, 2026 | 08:00)
- Int'l media provides large coverage of 14th National Party Congress's first working day (January 20, 2026 | 09:09)
- Vietnamese firms win top honours at ASEAN Digital Awards (January 16, 2026 | 16:45)
- ASEAN Digital Ministers' Meeting opens in Hanoi (January 15, 2026 | 15:33)






















 Mobile Version
Mobile Version