Vietnam begins clinical trial research for second Vietnamese-made COVID-19 vaccine
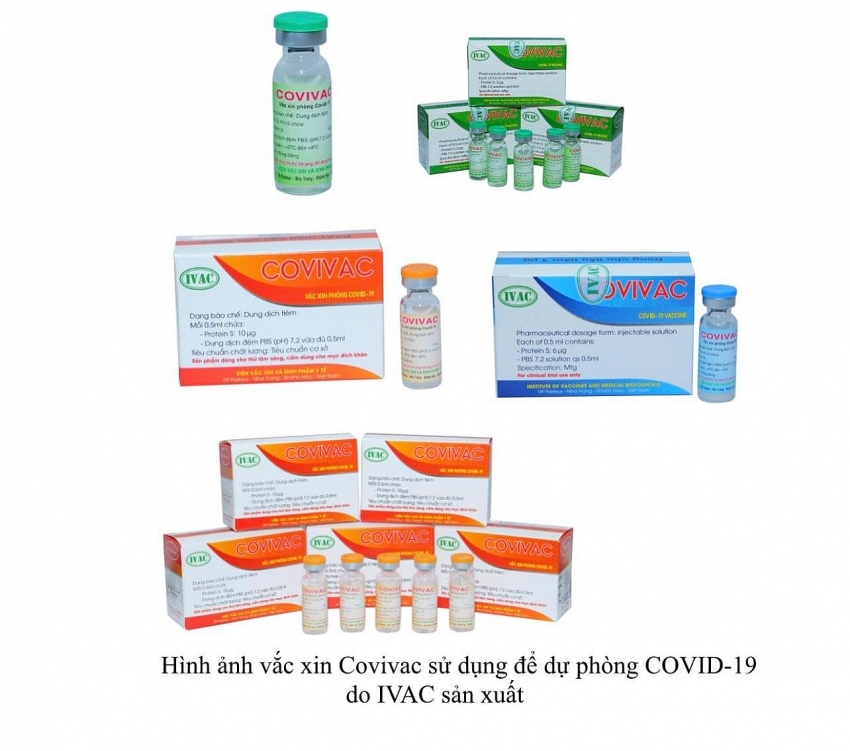 |
| COVIVAC vaccine is proceeding to human trials |
The COVIVAC vaccine has been developed by the Institute of Vaccines and Medical Biologicals (IVAC) since May 2020. IVAC has carried out a number of preclinical studies for the vaccine in India, the US, and Vietnam, with results showing that the injection is both safe and meets the relevant criteria to proceed to human trials.
Clinical trials will be carried out by the Central Institute of Hygiene and Epidemiology and Hanoi Medical University at Hanoi Medical University.
 |
| The launching ceremony of clinical trials for the COVIVAC vaccine |
The first phase will be conducted on 120 healthy volunteers who will be separated into five groups, receiving three doses of 1mcg, 3mcg, 10 mcg, and 1 mcg with a supplementary dose for placebo use, respectively.
The vaccination will consist of two injections administered 28 days apart, with the first volunteer set to be vaccinated in February.
After compiling research results from its initial phase, providing the vaccine meets safety standards and shows signs of strong immunity, the second phase will be conducted with a larger sample size.
COVIVAC vaccine is the second Vietnamese-made COVID-19 vaccine, following NanoCovax produced by Nanogen Pharmaceutical Biotechnology JSC that is now entering human trials.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Themes: Healthcare Platform
- PM outlines new tasks for healthcare sector
- Opella and Long Chau join forces to enhance digestive and bone health
- Hanoi intensifies airport monitoring amid Nipah disease risks
- Cosmetics rules set for overhaul under draft decree
- Policy obstacles being addressed in drug licensing and renewal
Related Contents
Latest News
More News
- US-Iran conflict affecting asset prices (March 04, 2026 | 09:17)
- Middle East tensions set to test VN-Index, boost energy stocks (March 02, 2026 | 17:15)
- Agency of Foreign Trade warns of trade disruption due to Middle East conflict (March 02, 2026 | 17:11)
- Spring Fair 2026 boosts domestic demand (March 02, 2026 | 16:30)
- Law on Investment takes effect (March 02, 2026 | 16:21)
- Ho Chi Minh City attracts nearly $980 million in FDI in early 2026 (March 02, 2026 | 10:57)
- Academic-policy network planned to support VIFC development (February 28, 2026 | 08:00)
- Businesses bouncing back after turbulent year (February 27, 2026 | 16:42)
- VinaCapital launches Vietnam's first two strategic-beta ETFs (February 26, 2026 | 09:00)
- PM sets five key tasks to accelerate sci-tech development (February 26, 2026 | 08:00)





 Tag:
Tag:
























 Mobile Version
Mobile Version