Pharmaceutical players seek expansion of rights in Vietnam
Having been present in Vietnam for over 30 years, Novo Nordisk Vietnam marked a significant milestone with its first shipment of products from Denmark to Vietnam as a fully recognised foreign-invested enterprise (FIE) in early July.
This milestone heralds a new era in the company’s expansion. In Vietnam, this event is crucial for multinational corporations (MNCs) in the pharma industry because a fully functional FIE enables a company to become an independent importer of its innovative products, expand local operations, and create new job opportunities for local talents.
 |
| Breakthrough pharma products have been made available in Vietnam over the past couple of years |
Erik Wiebols, vice president and general manager of Denmark-headquartered global healthcare company Novo Nordisk Vietnam, is optimistic about the company’s future performance in the local market following positive results.
“The company’s performance in the first half of the year had positive highlights. More patients are benefiting from our innovative diabetes and obesity medication, which brings better treatment and quality of life for people with chronic diseases,” he said.
Novo Nordisk has established a partnership with the Ministry of Health, the Danish Embassy, associations such as the Vietnam Association of Diabetes and Endocrinology and the Vietnam Pediatric Association, and top hospitals via an MoU signed in June 2024. One of the collaboration results was the issuance of the Type 1 diabetes guideline for sustainable Type 1 diabetes patient care.
“In the second half of this year, we remain optimistic about the opportunities to continue our mission to help more diabetes and obesity patients,” Wiebols said.
For Novo Nordisk, the future growth potential of the pharmaceutical market in Vietnam is highly promising, driven by several key factors and strategic initiatives: Vietnam’s economic growth, a rising prevalence of non-communicable diseases, the government’s comprehensive plan to develop the pharmaceutical industry by 2030 with a vision to 2045, and favourable policies and free trade agreements.
“The unmet needs in treatment are huge. We see great opportunities for serving the people living with diabetes and obesity in the country,” Wiebols said.
Increasing footprint
Gains are also being made by MNCs, particularly major pharmaceutical companies. Sanofi has made gains not only in the first half of 2024 but also since 2019 when it celebrated the first batch of pharmaceutical imports.
Emin Turan, country lead of Sanofi-Aventis Vietnam, said, “Since the beginning of 2024, we have achieved expected business results and are accelerating to meet this year’s goals. In addition, efforts in environmental, social, and governance criteria, talent development, and operations continue to be implemented with many positive results. In June, we introduced our advanced headquarters with a modern working style, which fosters better flexibility, trust, and prioritises the physical and mental wellbeing of employees.”
Globally, many of Sanofi’s breakthrough products have been launched and achieved positive results in various markets and will soon be available in Vietnam. “These results have been and will continue to be built upon our four key priorities: focusing on growth and prioritising our portfolio, leading with innovation and bringing transformative therapies to patients, accelerating operational efficiency, and reinventing how we work,” Turan added.
Having been in Vietnam for more than 70 years, Sanofi now has one manufacturing site serving domestic demands and export. Sanofi Vietnam is consistently among the top tax contributors among pharma firms. Its product portfolio includes vaccines, over-the-counter medicines, and prescription drugs.
Sanofi’s prescription drug category focuses on cardiovascular diseases, anticoagulation, anti-inflammation, blood pressure, and diabetes.
Similarly, GSK Vietnam said that in the first half of 2024, the company made significant progress in providing accessible medicine and vaccines in Vietnam.
“Through partnerships with medical associations, hospitals, healthcare professionals, and strategic partners, we are addressing infectious diseases like invasive meningococcal disease and chronic obstructive pulmonary disease with innovative vaccines and treatments. Our paediatric vaccines protect newborn babies against infectious diseases, while our general medicines treat various health conditions,” said Lien Pham, president of GSK Vietnam.
Over the past decade, GSK has protected over five million Vietnamese children from pneumococcal diseases, Pham said. It will continue to collaborate with health partners to prioritise disease prevention, especially in adults and older people who are more susceptible to infectious diseases.
“By proactively addressing health challenges and focusing on prevention, we can achieve broader benefits for health and socioeconomic factors,” Pham added.
In Vietnam, GSK continues to prioritise strengthening partnerships with partners like associations and hospitals, including the Vietnam Medical Association, National Phytopharma, and the Vietnam Vaccine Company, to increase its presence.
Great expectations
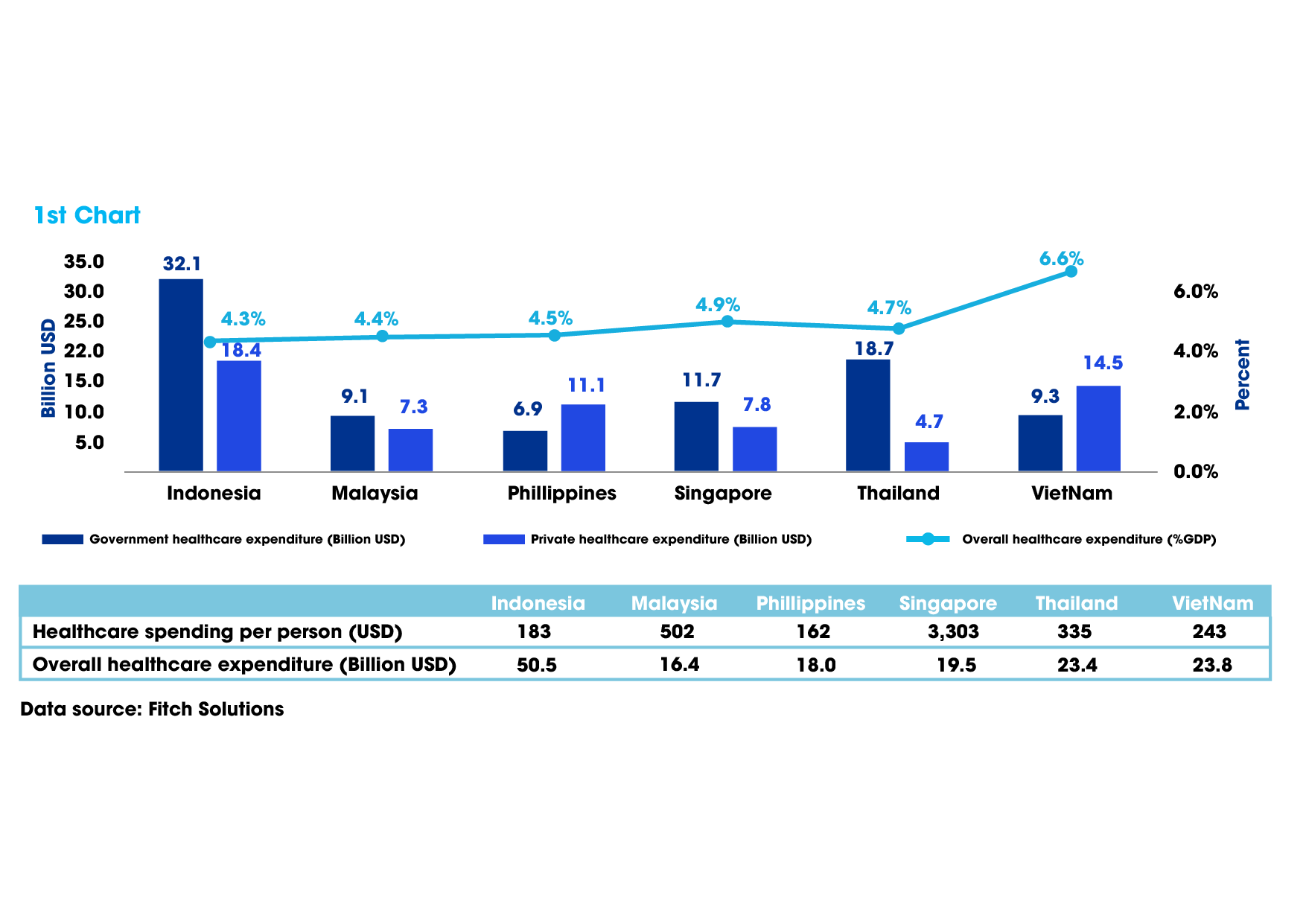
IBM Technology Group from the US foresees Vietnam’s medical and pharmaceutical market soaring to nearly $16.1 billion by 2026, positioning the nation as a pivotal contender for pharmaceutical advancement within Southeast Asia.
While making gains in the local market, MNCs are also seeking more simplified administrative procedures and incentives to expand further.
“The existing regulatory framework has posed some challenges for medicine importers and distributors during pandemic situations. Consequently, acquiring timely access to efficacious medicines for the prevention and treatment of diseases is a vital factor for the patients,” Turan of Sanofi-Aventis said.
In addition, the Law on Pharmacy 2016 partially broadens the rights of FIEs, but there are activities that FIEs do not have the right to perform. “For example, FIEs are not permitted to contact wholesalers or health establishments. If FIEs could directly exchange information with wholesalers or health establishments, including details on drug demand and supply plans, it would significantly reduce the risk of supply chain disruption and better ensure the availability of drugs for patients,” Turan added.
Draft amendments to the Pharmacy Law 2016 are being worked on, aiming to tackle issues and challenges identified during the enforcement of the current law, which have impacted the pharmaceutical production and business sectors. Key amendments involve adjustments to the rights and responsibilities of FIEs in the pharmaceutical industry, streamlining administrative procedures, and introducing policy incentives.
MNCs are now waiting for the October-November session of the National Assembly when the draft is expected to be adopted. Such corporations expect that these amendments will beneficially influence the pharmaceutical sector’s growth, broadening the range of activities qualified for investment incentives, enhancing technology, simplifying several administrative procedures, and accelerating the marketing authorisation (MA) process, allowing them to deliver and transport imported, processed, and technology-transferred drugs and pharmaceutical materials from their warehouses to wholesale facilities.
Darrell Oh, chairman of the Pharma Group, which represents the voice of 21 MNCs, said the drug registration provisions taking effect from 2025 are particularly critical. With thousands of MA approvals of essential medicines and vaccines expiring by the end of 2024, a well-aligned implementation plan for applying the new MA renewal provisions is crucial to ensure organisations can maintain supply to patients.
“The draft law is packed with exciting incentives to boost sector development. To make these a reality for investors, clear regulations in guiding documents will be crucial. This will provide the predictability needed for current projects and send a clearer, more positive signal to potential investors, both domestic and foreign,” Oh said.
 | Outlook for pharmaceutical stocks in 2024 At a macro level in which there is still complexity, the pharmaceutical industry is expected to continue witnessing positive business results in 2024. |
 | Imexpharm honoured with Vietnam Pharmaceutical Star award Imexpharm Pharmaceutical JSC proudly received the “Vietnam Pharmaceutical Star” award on May 17. |
 | Promoting Vietnamese pharmaceutical industry The Vietnamese pharmaceutical industry is seeing great advantages. |
 | Imexpharm announces interim results for H1 Imexpharm Corporation (IMP.VN), a pioneering leader in Vietnam’s pharmaceutical industry, has announced its interim results for H1. |
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Related Contents
Latest News
More News
- Vietnam sets ambitious dairy growth targets (February 24, 2026 | 18:00)
- Masan Consumer names new deputy CEO to drive foods and beverages growth (February 23, 2026 | 20:52)
- Myriad risks ahead, but ones Vietnam can confront (February 20, 2026 | 15:02)
- Vietnam making the leap into AI and semiconductors (February 20, 2026 | 09:37)
- Funding must be activated for semiconductor success (February 20, 2026 | 09:20)
- Resilience as new benchmark for smarter infrastructure (February 19, 2026 | 20:35)
- A golden time to shine within ASEAN (February 19, 2026 | 20:22)
- Vietnam’s pivotal year for advancing sustainability (February 19, 2026 | 08:44)
- Strengthening the core role of industry and trade (February 19, 2026 | 08:35)
- Future orientations for healthcare improvements (February 19, 2026 | 08:29)

 Tag:
Tag:


















 Mobile Version
Mobile Version