Enabling environment expected for international pharma firms
Certain amendments and supplements introduced in the draft hold particular significance for the development of foreign-invested enterprises (FIEs), promoting the activities of business cooperation, processing, and technology transfer between FIEs and domestic enterprises.
 |
| Trinh Luong Ngoc, partner, Vilaf |
The draft expands business and operational rights for importing FIEs, allowing them to sell pharmaceuticals and ingredients produced under outsourcing arrangements by the FIEs to wholesale establishments. Additionally, the draft permits importing FIEs to directly carry out delivery and transportation of their imported pharmaceuticals from their own warehouses to wholesale establishments.
The draft also expands wholesale and transportation rights for manufacturing FIEs for outsourced products in Vietnam, allowing them to distribute and transport pharmaceuticals and ingredients produced under outsourcing arrangements within the country.
Allowing importing and manufacturing FIEs to sell products under outsourcing arrangements, and granting them greater autonomy in the delivery and transportation of pharmaceuticals and ingredients, will promote outsourcing and tech transfer, and ensure the quality of products during delivery and transportation, which is crucial for safeguarding public health. It also helps minimise costs and save time by eliminating complicated on-the-spot export-import procedures. As a result, high-quality products can be quickly brought to market, enhancing business efficiency for both domestic and foreign enterprises, while ensuring that the public can access advanced pharma in a timely manner and at reasonable costs.
The draft also simplifies administrative procedures for the registration of pharmaceuticals and ingredients, primarily by reducing documentation requirements. Additionally, it empowers the Minister of Health to evaluate, recognise, and adopt Good Manufacturing Practices standards from advanced countries, thereby facilitating a smoother application and renewal process for marketing authorisations (MAs).
From a legal perspective, the draft amendment has introduced many positive changes. However, to enhance the effectiveness and transparency of pharma management and business activities in Vietnam, some proposals are recommended.
Firstly, the draft has made improvements in reducing paperwork. However, we propose setting a shorter timeframe for processing applications for drug MAs compared to the current 12-month period. Shortening this duration will enable businesses to bring products to market faster and optimise their business operations.
The second aspect is to abolish the procedure for renewal of MAs and replace it with notification and strengthened post-market surveillance. Although the MoH has evaluated and proposed a simplified procedure and dossier for renewal, we suggest abolishing the renewal procedure, requiring only a notification from enterprises and transitioning to enhanced post-market surveillance.
This option aligns with the government’s spirit of simplifying administrative procedures, creating a more favourable environment for businesses, reducing costs, increasing stability in business operations, and simultaneously attracting foreign investment while improving competitive capacity.
Through our legal consulting experience with businesses in the pharmaceutical industry, we have observed several common challenges faced by enterprises due to inconsistent regulations and complex administrative procedures.
The first involves drugs manufactured under outsourcing contracts by FIEs. Under the current Law on Pharmacy, when FIEs outsource drug manufacturing, they need to perform on-the-spot export and import procedures before being able to sell to wholesale establishments. This process is not only time-consuming and costly, but also hinders the objective of delivering high-quality medicines to patients in the shortest possible time.
Furthermore, pharma companies often encounter difficulties when their MAs expire, but the renewal process is delayed due to complicated assessment procedures. Under current regulations, if the licence is not renewed in time, enterprises must temporarily halt the circulation of the product, causing disruptions in the supply chain and negatively impacting end-users.
To make regulations more favourable for businesses, in the short term, improvements should be further made in the certain areas. Vietnam needs to strengthen inspection measures and ensure consistent and effective enforcement of legal regulations to protect the legitimate rights and interests of FIEs. This includes strict inspections, handling of intellectual property rights violations, and establishing a robust coordination mechanism between regulatory agencies and businesses.
Accelerating the simplification of procedures for the registration and renewal of MAs, enabling businesses to quickly register and launch their products in the market, would promote competition and ensure earlier access to medicine for the public. Reforming administrative procedures for investment to shorten the licensing and implementation timeline for large-scale projects would also attract more foreign investors and promote economic development.
In the long term, it should expand business rights for FIEs, allowing them to fully participate in the distribution sector; and establish comprehensive policies to encourage research and development activities, promoting technology transfer to domestic companies. This will improve the quality of pharmaceuticals and reduce costs, supporting sustainable development and enhancing the competitiveness of the domestic industry.
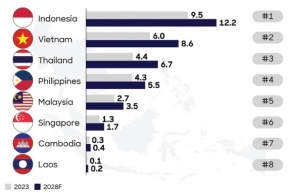 | Pharma to experience robust growth Vietnam healthcare market size is estimated to reach $23.7 billion and grow at an average rate of 7.5 per cent annually from 2023 to 2028. Healthcare expenditure per capita is also projected to increase from $237 to $328 in that time. |
 | Competition within ASEAN for pharma investment With an ageing population, rising disease burden, and advancements in healthcare science, the global pharmaceutical market has seen significant growth in recent years. In 2023, the total market was estimated at $1.6 trillion, an increase in over $100 billion compared to 2022. As one of the largest and fastest-growing industries, the sector drives healthcare innovation globally. |
 | Incentives ahead for pharma industry Vietnam’s pharmaceutical industry has reached level three under the World Health Organisation’s classification, as the country can now produce some basic raw materials. However, it has yet to fully develop raw material production, as it cannot compete with imported materials. |
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Related Contents
Latest News
More News
- Citi economists project robust Vietnam economic growth in 2026 (February 14, 2026 | 18:00)
- Sustaining high growth must be balanced in stable manner (February 14, 2026 | 09:00)
- From 5G to 6G: how AI is shaping Vietnam’s path to digital leadership (February 13, 2026 | 10:59)
- Cooperation must align with Vietnam’s long-term ambitions (February 13, 2026 | 09:00)
- Need-to-know aspects ahead of AI law (February 13, 2026 | 08:00)
- Legalities to early operations for Vietnam’s IFC (February 11, 2026 | 12:17)
- Foreign-language trademarks gain traction in Vietnam (February 06, 2026 | 09:26)
- Offshore structuring and the Singapore holding route (February 02, 2026 | 10:39)
- Vietnam enters new development era: Russian scholar (January 25, 2026 | 10:08)
- 14th National Party Congress marks new era, expands Vietnam’s global role: Australian scholar (January 25, 2026 | 09:54)

 Tag:
Tag:



















 Mobile Version
Mobile Version