Fresh rules to tackle pharma misconduct
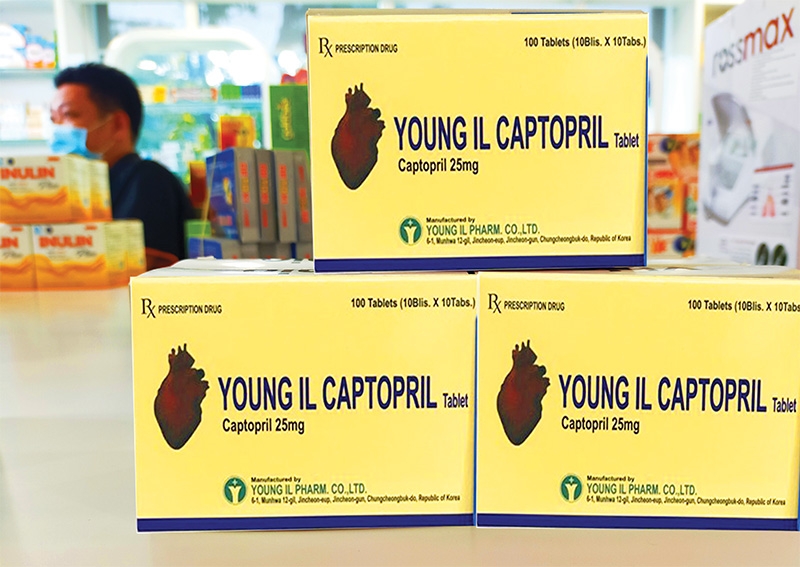 |
| Pharma groups will face tighter consequences under a new decree, photo Le Toan |
The Drug Administration of Vietnam (DAV) under the Ministry of Health has revealed a number of domestic and international companies violating administrative rules.
The DAV imposed a fine of VND70 million ($3,000) on South Korea’s Young Il Pharm for producing Young II Captopril tablets that are not qualified for Level 2 in line with prevailing rules in Article 39 of 2013’s Decree No.176/2013/ND-CP on penalties for administrative violations against medical laws.
This is not the first time that the South Korean pharma firm has received a fine. In January, a similar punishment was also imposed on it when the DAV decided to take back Captopril tablets for failing to meet quality requirements. This tablet was imported by Saigon Pharmaceutical Company.
The South Korean group has a representative office in Ho Chi Minh City, focusing on studying the market, and seeking and promoting business opportunities. Notably, it is one of the international pharma giants striving to get the most sought-after Good Manufacturing Practice recognition for the Penmix Ltd. manufacturing site to venture further into the hospital system and the ethical drugs channel.
It has not been an easy path for Young Il Pharm and others to qualify for better standards, and has been asked to supplement more documents as requested by the DAV.
Similarly, USV Private, a multinational pharmaceutical and biotechnology company based in Mumbai city of India, was fined “for making small changes without informing authorised agencies”. The company established its representative office in Vietnam in 2016, which is responsible for seeking investment opportunities for its representative MI Pharma Private Limited in the country.
Also on the fine list is Vietnamese-run pharmaceutical and medical equipment company Armepharco, which received a fine for violating drug trading activities as stated in the certificate of eligibility for drug businesses.
Armepharco specialises in making drugs that do not contain beta-lactam antibiotics, and others. The drugmaker has been operating as a joint-stock company since 2010 with a vision to become Vietnam’s number-one pharmaceutical group.
The violations by these firms and many more have triggered some concerns over their code of ethics and whether there have been any victims.
Violations of drug quality and related matters are not a rare issue in the lucrative pharmaceutical industry, with the number increasing on an annual basis. Last year, a number of guilty parties were also announced, including major names such as Korea E-Pharm, Windlas Biotech of India, Pharmix Corporation of South Korea, and Tada Pharma among others.
Industry insiders said that because of humble punishments, companies are often willing to violate some rules to gain higher profits. The Vietnamese drug market posted turnover of $5.2 billion in 2017, according to data from London-based market researcher Business Monitor International. This was up about 10 per cent on-year and is expected to continue double-digit growth over the next five years. Moreover, Vietnam’s drug spending per capita rose by 10.6 per cent on-year to about $53 in 2018, and is forecast to rise further in the near future.
However, their future dark intentions might be deterred as Decree No.117/2020/ND-CP dated September 28, governing fines in the healthcare sector, will take effect from November 15. The new decree, which will replace Decree 176, will feature new toughest-ever rules including suspension of violation-related business activities for 1-3 months if mobile drug retailing establishments fail to satisfy the conditions as ruled, and suspension of violation-related business activities for 6-12 months for forging papers in dossiers announcing business establishment.
In addition, some guilty parties may even have their certificates of eligibility for pharmacy temporarily revoked for up to two years.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
 Tag:
Tag:
Related Contents
Latest News
More News
- Vietnam financial markets on the rise amid tailwinds (February 11, 2026 | 11:41)
- New tax incentives to benefit startups and SMEs (February 09, 2026 | 17:27)
- VIFC launches aviation finance hub to tap regional market growth (February 06, 2026 | 13:27)
- Vietnam records solid FDI performance in January (February 05, 2026 | 17:11)
- Manufacturing growth remains solid in early 2026 (February 02, 2026 | 15:28)
- EU and Vietnam elevate relations to a comprehensive strategic partnership (January 29, 2026 | 15:22)
- Vietnam to lead trade growth in ASEAN (January 29, 2026 | 15:08)
- Japanese business outlook in Vietnam turns more optimistic (January 28, 2026 | 09:54)
- Foreign leaders extend congratulations to Party General Secretary To Lam (January 25, 2026 | 10:01)
- 14th National Party Congress wraps up with success (January 25, 2026 | 09:49)





















 Mobile Version
Mobile Version