Vietnam completes first phase of Nano Covax human trials
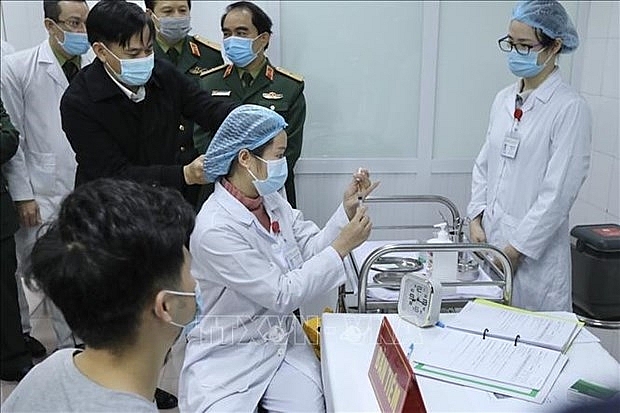 |
| Vietnam has completed the first phase of the human trials of Nano Covax. (Photo: VNA) |
Hanoi – Vietnam has completed the first phase of the human trials of Nano Covax, a homegrown COVID-19 vaccine, with the participation of 60 volunteers, the Vietnam Military Medical University said on February 8.
The last 22 volunteers received the second shots, with two receiving the 50mgc dose, and 20 getting the 75mgc dose.
Experts said Nano Covax has proven safe and effective against the coronavirus SARS-CoV-2, even its new variant.
Most of the volunteers are in stable conditions after vaccination. Only few had light injection site pain and fever that disappeared after one to two days.
The objective of the first phase is to evaluate the safety of the vaccine, developed and manufactured by HCM City-based Nanogen Pharmaceutical Biotechnology JSC.
Nearly 400 people have registered for the second phase that is expected to last for six months, during which 560 volunteers will be injected.
The third phase will see the engagement of more than 10,000 volunteers, beginning from August.
If the result is good, Vietnam would administer the vaccine to the public in early 2022.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
 Tag:
Tag:
Related Contents
Latest News
More News
- Congratulations from VFF Central Committee's int’l partners to 14th National Party Congress (January 25, 2026 | 09:46)
- List of newly-elected members of 14th Political Bureau announced (January 23, 2026 | 16:27)
- 14th Party Central Committee unanimously elects To Lam as General Secretary (January 23, 2026 | 16:22)
- List of members of 14th Party Central Committee announced (January 23, 2026 | 09:12)
- Highlights of fourth working day of 14th National Party Congress (January 23, 2026 | 09:06)
- Press provides timely, accurate coverage of 14th National Party Congress (January 22, 2026 | 09:49)
- Press release on second working day of 14th National Party Congress (January 22, 2026 | 09:19)
- Minister sets out key directions to promote intrinsic strength of Vietnamese culture (January 22, 2026 | 09:16)
- 14th National Party Congress: Renewed momentum for OVs to contribute to homeland (January 21, 2026 | 09:49)
- Party Congress building momentum for a new era of national growth (January 20, 2026 | 15:00)






















 Mobile Version
Mobile Version