Roche total solutions to fight against COVID-19
Facing the unprecedented developments of the COVID-19 outbreak and with many new COVID-19 community infections, the Vietnamese healthcare sector is supporting to accelerate testing capabilities. As of August 10, there were 70 labs in Vietnam allowed to perform confirmatory testing for COVID-19 with the capacity of about 31,000 patient samples a day.
Diagnostics plays an essential role as its results are influencing over 60 per cent of clinical decision-making while accounting for only about 2 per cent of total healthcare spending. Currently, many people are joining the call for health declaration, testing registration, and interested in getting tested to feel secure in order to go to work and school.
Hospitals, district, ward and commune-level health centres, and even the private health sector are actively looking for reliable testing solutions for their staff to preserve the healthcare workforce and complete medical examinations and treatment for the community. Many organisations and agencies are also considering assessing their employees' health status to craft business recovery and economic development plans, among others.
| Roche immunology instruments can perform the Anti-SARS-CoV-2 antibody test in approximately 18 minutes, with capacity up to hundreds of tests an hour, depending on the type of analyser. |
“Every reliable test on the market serves a specific healthcare purpose, which is to help us overcome this pandemic. Roche is working closely with health authorities to promptly deliver high-quality tests to Vietnamese people,” said Qadeer Raza, general manager of Roche Diagnostics Vietnam.
“To actively join hands with the Vietnamese government to prevent the outbreak, Roche Diagnostics Vietnam donated more than 5,000 tests for Anti-SARS-CoV-2 antibody and RT-PCR tests to the Ministry of Health. Besides, our employees are working 24/7 to support frontline hospitals in outbreak prevention by ensuring that the diagnostics instruments are installed and operating smoothly during this critical time," Raza noted.
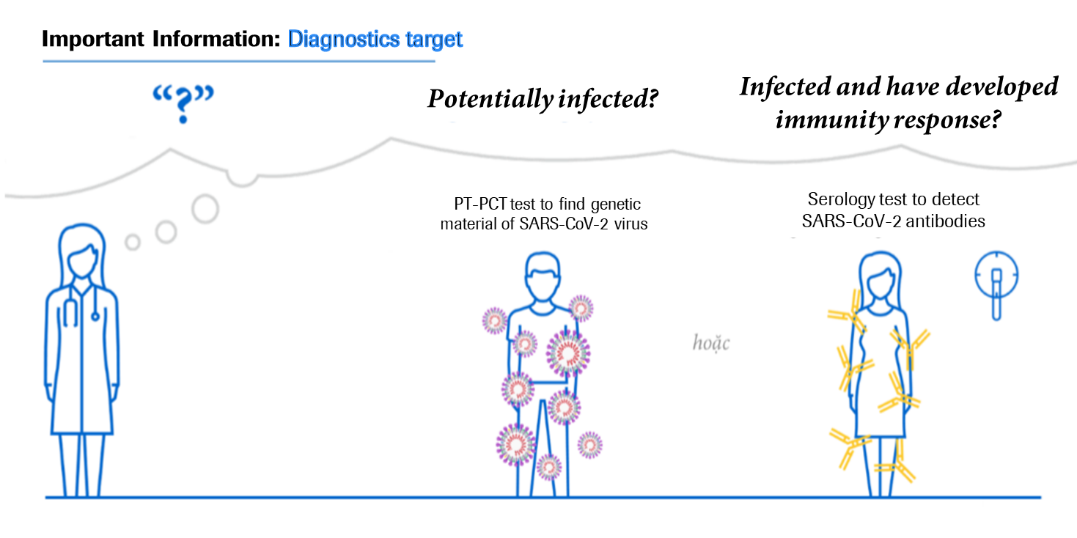
In Vietnam, Roche Diagnostics Vietnam provides two types of tests: RT-PCR molecular diagnostic test and Anti-SARS-CoV-2 antibody test. These two diagnostics methods have been assessed to support COVID-19 management.
RT-PCR test confirms whether the patient is infected with SARS-CoV-2 or not, whereas serological tests for antibodies help determine whether a person has ever been infected, from there determining the infection rate and the community’s status. Let us have a closer look on the tests’ functions.
- Realtime PCR test (RT-PCR)
In 1983, Kary Mullis, PhD, a scientist at Cetus Corporation, conceived PCR as a method to copy DNA and synthesise large amounts of a specific targeted DNA. With this innovation of PCR technology, he and Michael Smith were awarded the Nobel Prize in Chemistry back in 1993 for their contributions to the development of methods within DNA-based chemistry.
In 1991, Roche acquired the rights to PCR from Cetus and invested in refining the science for use in molecular diagnostics to detect diseases. Roche Molecular Diagnostics has not only defined and refined PCR technology, but has remained the clear leader of this technology.
PCR technology has been applied and now developed the test which detects the genetic materials of SARS-CoV-2 in respiratory tract secretions, which helps to confirm the infection. The diagnosis of patients infected with SARS-CoV-2 enables patient treatment and quarantine plans as well as avoiding the spreading of infection to other contacts.
- Anti-SARS-CoV-2 antibody test
Antibody testing is considered the next critical step in the fight against COVID-19. The Anti-SARS-CoV-2 antibody test is an in-vitro test that uses serum or plasma taken from human blood samples to detect antibodies that can determine the body's immune response to SARS-CoV-2. This test can be used in epidemiological research to better understand the spread of the disease. It can also be used in conjunction with molecular biology assays to confirm the diagnosis of COVID-19-suspected patients.
Through a blood sample, the test can detect antibodies to the SARS-CoV-2 virus, indicating that the person has been infected with the SARS-CoV-2 virus and that the body has produced antibodies against the virus. Roche immunology instruments can perform the Anti-SARS-CoV-2 antibody test in approximately 18 minutes, with capacity up to hundreds of tests an hour, depending on the type of analyser.
Anti-SARS-CoV-2 antibody test for antibodies helps determine the infection rate and community immune status. High specificity is required for the Anti-SARS-CoV-2 antibody test to determine which patients have truly developed an immune response to COVID-19, while he or she can still be infected with COVID-19. Roche’s serology test has a specificity greater than 99.8 per cent and sensitivity of 100 per cent.
 |
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Themes: Together We Win
- Greater Mekong Subregion executives to discuss sustainable tourism
- TCPVN donates 1,200 medicine bags to COVID-19 patients in southwest
- AB InBev supports orphans with scholarships amid COVID-19
- Evaluating the reach of support in turbulent times
- Gamuda Land grants “Back to School” scholarships to support disadvantaged students
Related Contents
Latest News
More News
- Vietnam Television launches third 'Song Sau Lu' project for 2025 (December 15, 2025 | 08:00)
- Closing workshop highlights five-year impact of Fair for All project (December 12, 2025 | 16:22)
- Stakeholders mobilised before new child safety rules take effect (December 10, 2025 | 09:00)
- Vietnam receives emergency international relief as regional flooding intensifies (December 04, 2025 | 15:11)
- AmCham scholarships awarded to students (December 02, 2025 | 16:46)
- Vietjet flights carry love to devastated central region (November 28, 2025 | 11:35)
- SCG Sharing the Dream supports Vietnam’s youth and sustainable development goals (November 28, 2025 | 10:55)
- Siemens Caring Hands donates $34,700 for disaster relief in Vietnam (November 26, 2025 | 20:25)
- Ireland extends support for the Resilience First initiative (November 26, 2025 | 15:24)
- South Korea funds IOM relief for Vietnam’s typhoon-affected communities (November 24, 2025 | 15:33)

 Tag:
Tag:


























 Mobile Version
Mobile Version