Veterinary drugmakers seek time and cost-saving policies
The drug producers await a National Assembly (NA) decision on passing the law amending and supplementing some articles of the Law on Standards and Technical Regulations. When the amended law is approved, there will be shortened orders and procedures on clarifying veterinary drug conformity, which is likely to save producers a great deal of money a year.
 |
| Veterinary drugmakers seek time and cost-saving policies, illustration photo/ Source: freepik.com |
“The cost of sample analysis and testing alone is huge,” said Nguyen Nhu So, chairman of the board at Dabaco Group and chairman of the Vietnam Animal Feed Association.
“For example, veterinary medicine ranges from $82-165 per product and $415-830 per vaccine product during the accreditation assessment. In addition, the testing also is repeated during the circulation of three years of the products,” So said. “If a facility has hundreds of veterinary drugs and thousands of products, the cost for completing procedures for declaration of conformity skyrockets.”
In late March, the Ministry of Agriculture and Rural Development (MARD) reported to the government about inadequacies in declaring veterinary drug conformity. The MARD proposed to the government to allow the ministry to amend rules regulating the management of veterinary drugs according to the compacted procedures.
“These amendments will ensure the declaration of veterinary drug conformity according to regulations, which does not require annual sampling to monitor product quality assessment, and does not require production process assessment, contributing to reducing production and business costs,” Deputy Minister of Agriculture and Rural Development Phung Duc Tien said.
The MARD also asked the government to issue a document which requires temporarily suspending or not sanctioning administrative violations for the production, import, and circulation of veterinary drugs that have been granted a certificate of circulation in Vietnam but have not declared conformity since February 14. These temporary suspensions may be slated until the NA passes the law in question, and it takes effect.
“In the long term, the MARD proposed that the government consider requesting the NA to amend and supplement the Law on Standards and Technical Regulations in the direction of excluding veterinary drugs from having to declare conformity,” Tien said.
In the last two weeks, livestock industry groups such as the Vietnam Livestock Association, the Vietnam Large Cattle Breeding Association, the Vietnam Poultry Farming Association, and the Vietnam Animal Feed Association submitted a petition to the prime minister and the chairman of the NA to address issues in the industry.
The associations said that the production of veterinary medicine is a conditional business, and so production and trading establishments of these items must meet the conditions assessed and appraised by state agencies for issuance of certificates before production, and annually take the agency’s maintenance supervision assessment.
In addition, there are periodic and irregular inspections, as well as inspections by other authorities of the ministry or locality. Thus, the assessment and declaration of conformity by various bodies are duplicated and overlapping.
“In general, the declaration of conformity is formalistic and confrontational because veterinary medicine facility often has hundreds of products registered for circulation. However, the certification organisations just evaluate the production process of a few products and just take some samples for testing,” the associations wrote. “Meanwhile, in reality, many producers also mixed and prepared available samples by themselves so that certification organisations can take these samples for analysis, which means that the declaration of conformity is invaluable work.”
While waiting for the law to be amended, the associations expect the temporary suspension of the declaration of conformity for veterinary medicine products, or otherwise must shoulder a huge financial burden.
 | Drugmakers prepare to offer home vaccines to the world As Vietnam is stepping up its homegrown vaccine development, there is a high chance for Vietnamese vaccine producers to expand their presence in the global pharmaceuticals market. |
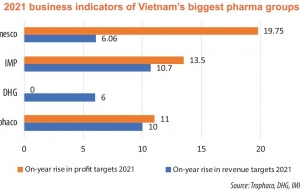 | Drug giants gaining with fresh products While Vietnam’s drugmakers are heading to develop new products to increase efficiency and sustain sustainable growth, competition from imported drugs is making their road tougher. |
 | Drugmakers yearning for MA clarity Despite the government’s strong request for facilitation of marketing authorisation renewal, foreign drugmakers are still waiting for the renewal of expired authorisations, triggering concerns over a possible future shortfall of drugs. |
 | Drugmakers build on early 2023 gains While making profits in 2022 from market recovery, Vietnamese drug giants are restructuring to strengthen sales and new products to gain market share, both at home and abroad. |
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Related Contents
Latest News
More News
- Masan Consumer names new deputy CEO to drive foods and beverages growth (February 23, 2026 | 20:52)
- Myriad risks ahead, but ones Vietnam can confront (February 20, 2026 | 15:02)
- Vietnam making the leap into AI and semiconductors (February 20, 2026 | 09:37)
- Funding must be activated for semiconductor success (February 20, 2026 | 09:20)
- Resilience as new benchmark for smarter infrastructure (February 19, 2026 | 20:35)
- A golden time to shine within ASEAN (February 19, 2026 | 20:22)
- Vietnam’s pivotal year for advancing sustainability (February 19, 2026 | 08:44)
- Strengthening the core role of industry and trade (February 19, 2026 | 08:35)
- Future orientations for healthcare improvements (February 19, 2026 | 08:29)
- Infrastructure orientations suitable for a new chapter (February 19, 2026 | 08:15)

 Tag:
Tag:


















 Mobile Version
Mobile Version