Vaccination rush rolls ahead in target to regain normalcy
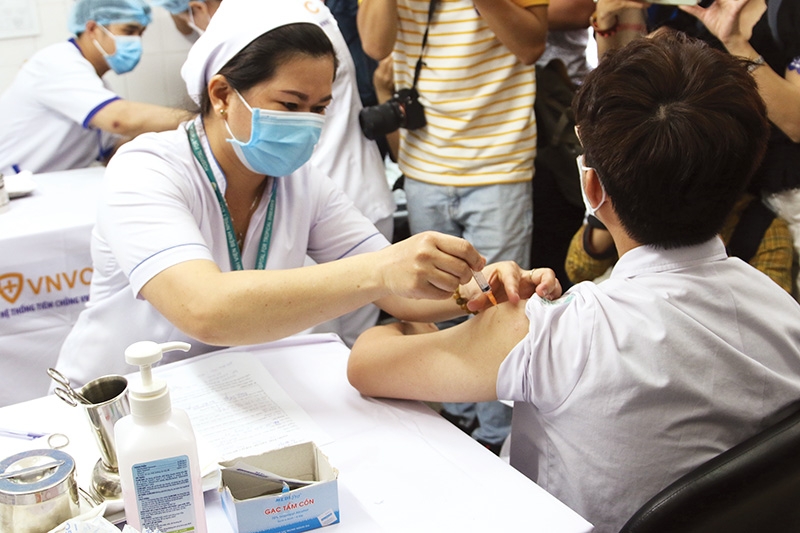 |
| Approved vaccines for COVID-19 will overwhelmingly offer benefits for life to get back to normal. Photo: Le Toan |
The Ministry of Health (MoH) on March 18 issued temporary guidance on examinations before vaccinations with the COVID-19 vaccine by AstraZeneca, aiming to classify groups of eligible recipients for a shot to increase safety.
Vietnamese Minister of Health Nguyen Thanh Long said, “Vietnam will continue the vaccinations with AstraZeneca’s product, as there have been no reports of blood clots after the concluded vaccinations.” The minister also asked authorised health units to provide training for staff.
Meanwhile, more than a dozen countries, mostly in Europe, had suspended the use of the AstraZeneca vaccine over fears the shot may have caused some recipients to develop blood clots. However, Italy, France, Germany, and several other countries resumed administering the jabs from last Friday after Europe’s medicines regulator said the vaccine was “safe and effective” and its benefits outweighed its risks. Portugal will resume on Monday, Spain and the Netherlands later this week, while Sweden’s public health agency said it would take “a few days” to decide.
An AstraZeneca spokesperson told VIR, “The safety of all is our first priority. We are working with national health authorities and European officials and look forward to their assessment later this week.”
“Around 17 million people in the European Union and United Kingdom have now received our vaccine, and incidents with reported blood clots in this group are lower than what was expected among the general population,” the spokesperson added.
Vietnam started COVID-19 vaccinations in March 8 using 117,000 doses of vaccines provided by AstraZeneca. By March 17, over 24,000 healthcare workers were vaccinated. The country has reported its first case of serious anaphylaxis, and some side effects like fever, swelling and pain at the site of injection, headaches, nausea, joint pain, and diarrhea.
According to the National Institute of Hygiene and Epidemiology, the number of people suffering side effects is within the expected level.
In February, when the World Health Organization (WHO) listed the AstraZeneca/Oxford vaccine for emergency use, it was found to be 76 per cent effective against the original coronavirus after the first dose. After the second dose, the efficacy rises to 82 per cent. This vaccine has been hailed for its affordability and convenient distribution and is expected to be a mainstay in protection for much of the world.
WHO leaders also met to review the available safety data on the vaccine, although they repeatedly expressed confidence in its safety. WHO chief Tedros Adhanom Ghebreyesus has said there was no evidence of harmful side effects so far.
Similarly, the European Medicines Agency (EMA) has said there is currently no indication that the vaccine has caused serious conditions, which are not listed as side effects of the AstraZeneca vaccine. The position of EMA’s Pharmacovigilance Risk Assessment Committee is that the vaccine’s benefits continue to outweigh its risks and the vaccine can continue to be administered while investigation of cases of thromboembolic events is ongoing.
Amid the concern, many countries continue to use the AstraZeneca vaccine. To boost confidence for the general public, Thai Prime Minister Prayuth Chan-ocha became the first person to be inoculated with the vaccine in the country on March 16. And Thailand’s health minister has also said that the rollout would resume after many countries confirmed there were no blood clot issues.
To increase supply for countries amid efforts of putting an end to the pandemic, the WHO on March 12 approved the Johnson & Johnson (J&J) vaccine for emergency use, paving the way for the one-shot dose to be used as part of the United Nation’s global vaccine distribution efforts.
It is the third COVID-19 vaccine after the two-shot regimens of Pfizer-BioNTech and AstraZeneca to receive backing from the WHO. According to J&J, the company expects to produce up to three billion doses of the vaccine next year, after previously pledging to deliver one billion globally by the end of 2021.
Studies have shown the J&J version to be 66 per cent effective in protecting against any moderate to severe illness and 85 per cent effective against severe cases of COVID-19, and is supposed to completely prevent hospitalisations and related deaths four weeks after inoculation. Some comparisons have been made between the J&J vaccine and others. According to experts, when the vaccines were approved, Pfizer’s efficacy was at 95 per cent, while Moderna’s was 94.1 per cent.
“All vaccines offer essentially the same effectiveness in terms of severe COVID-19 hospitalisation and death,’’ said Veronica McNally, a member of the Center for Disease Control’s Advisory Committee on Immunization Practices. “There are going to be people who are very enticed by the fact that there is only one shot – people who want to be vaccinated quickly and those who do not want to return or cannot return for a second dose.’’
Vietnam is still negotiating with manufacturers in the United States, Russia, and other countries as part of an effort to diversify vaccine sources. The country aims to obtain a total of 150 million doses this year to cover 70 per cent of its population. Russia has thus far donated 1,000 doses of the Sputnik V vaccine to Vietnam, while the US pharmaceutical firm Pfizer may provide 30 million doses within 2021.
| World Health Organisation on AstraZeneca COVID-19 vaccine safety Some countries in the European Union have temporarily suspended use of the AstraZeneca COVID-19 vaccine as a precautionary measure based on reports of rare blood coagulation disorders in persons who had received the vaccine. Other countries in the EU – having considered the same information - have decided to continue using the vaccine in their immunisation programmes. Vaccination against COVID-19 will not reduce illness or deaths from other causes. Thromboembolic events are known to occur frequently. Venous thromboembolism is the third-most common cardiovascular disease globally. In extensive vaccination campaigns, it is routine for countries to signal potential adverse events following immunisation. This does not necessarily mean that the events are linked to vaccination itself, but it is good practice to investigate them. It also shows that the surveillance system works and that effective controls are in place. The World Health Organisation (WHO) is in regular contact with the European Medicines Agency and regulators around the world for the latest information on COVID-19 vaccine safety. The WHO COVID-19 Subcommittee of the Global Advisory Committee on Vaccine Safety is carefully assessing the latest available safety data for the AstraZeneca vaccine. Once that review is completed, the WHO will immediately communicate the findings to the public. At this time, the WHO considers that the benefits of the AstraZeneca vaccine outweigh its risks and recommends that vaccinations continue. |
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Themes: Healthcare Platform
- Hanoi intensifies airport monitoring amid Nipah disease risks
- Cosmetics rules set for overhaul under draft decree
- Policy obstacles being addressed in drug licensing and renewal
- Sanofi, Long Chau Pharmacy relaunch medicine blister pack collection initiative
- Takeda Vietnam awarded for ongoing support of Vietnam’s sustainability efforts
Related Contents
Latest News
More News
- Foreign leaders extend congratulations to Party General Secretary To Lam (January 25, 2026 | 10:01)
- 14th National Party Congress wraps up with success (January 25, 2026 | 09:49)
- Congratulations from VFF Central Committee's int’l partners to 14th National Party Congress (January 25, 2026 | 09:46)
- 14th Party Central Committee unanimously elects To Lam as General Secretary (January 23, 2026 | 16:22)
- Worldwide congratulations underscore confidence in Vietnam’s 14th Party Congress (January 23, 2026 | 09:02)
- Political parties, organisations, int’l friends send congratulations to 14th National Party Congress (January 22, 2026 | 09:33)
- Press release on second working day of 14th National Party Congress (January 22, 2026 | 09:19)
- 14th National Party Congress: Japanese media highlight Vietnam’s growth targets (January 21, 2026 | 09:46)
- 14th National Party Congress: Driving force for Vietnam to continue renewal, innovation, breakthroughs (January 21, 2026 | 09:42)
- Vietnam remains spiritual support for progressive forces: Colombian party leader (January 21, 2026 | 08:00)

 Tag:
Tag:




















 Mobile Version
Mobile Version