Overhaul anticipated for transfer of pharma tech
New changes in technology transfer as part of amendments to Vietnam’s Law on Pharmacy (LoP) are being sought after by the business community, at the same time as being discussed at the National Assembly (NA).
 |
Nguyen Thanh Lam, deputy director of the Drug Administration of Vietnam, said that the development of the pharmaceutical industry needs mechanisms to meet the new development situation.
“The LoP 2016 focuses on the development of generic drugs, raw materials for drug production, and basic materials. The draft amendments add regulations on attracting investment in research and development, incentives for developing innovative and high-tech drugs, and those with special dosage forms, especially vaccines and biological products that we previously have to import,” Lam added.
“Regulations are also being drafted on ensuring consumption output for drugs made in Vietnam, especially those produced by foreign-invested enterprises (FIEs) in the country,” he clarified. “The rights for FIEs are planned to be expanded if they invest in manufacturing processing, and tech transfer for manufacturing of innovative, branded, and high-tech drugs in Vietnam.”
The amendments, welcomed by domestic and multinational corporations, will strongly facilitate business activities, if approved.
Nguyen Thuy Anh, chairwoman of the National Assembly Committee for Social Affairs, said, “Vietnam has a lot of potential to develop the pharmaceutical industry, but domestic pharmaceutical businesses have not taken advantage due to lack of resources and limited technical and technological level. Meanwhile, large foreign investors are ready to invest in large projects.”
It is necessary to tap into the existing strengths and potential of the pharmaceutical industry with feasible mechanisms and support in terms of capital, human resource training, scientific research, and technology transfer, Anh added, while attracting foreign investment in areas where Vietnam is weak, such as the production of advanced treatment products and high-value biological drugs.
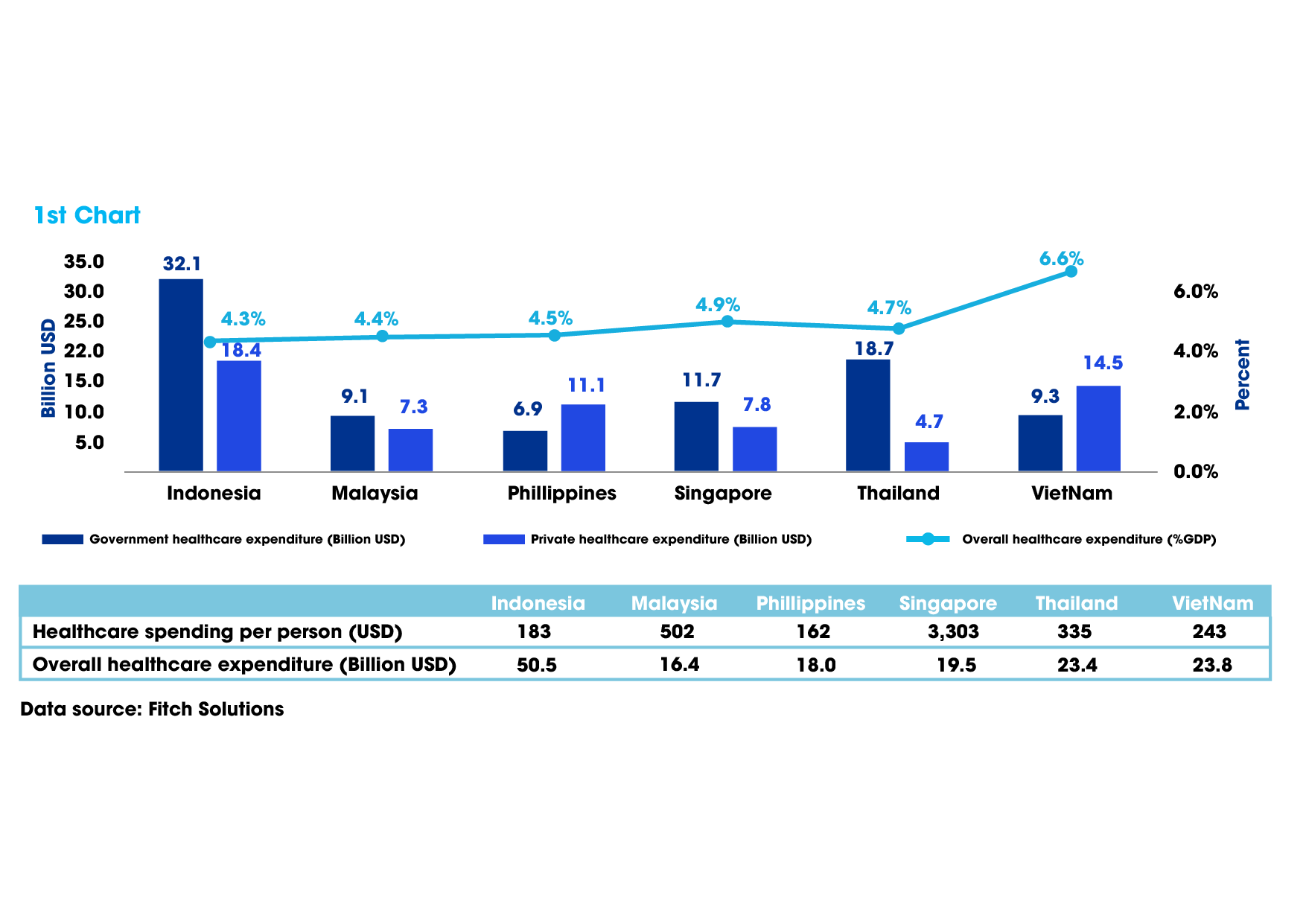
According to experts, about 90 per cent of medical ingredients for producing western medicines must be imported. One of the reasons is that the LoP 2016 and legal documents related to investment incentives have not supported the development of medicinal raw materials, and still cause difficulties in attracting large corporations to transfer technology.
Vinapharm general director Han Thi Khanh Vinh said draft amendments to the LoP should have investment incentives for local production of generic drugs and corporate income tax incentives. “Investment incentives for firms and projects in the pharma industry should be issued without being considered with the investment scale as stated in the current rules,” she said.
Under the current regulation, FIEs acting as contract acceptors are permitted to sell contract manufacturing organisations (CMOs) or toll manufacturing/technology transfer products they produce. However, FIEs acting as contract givers can only sell CMO products through registered wholesalers, typically local pharmaceutical companies, who subsequently distribute them to hospitals and pharmacies.
“Alternatively, FIEs must perform on-spot export and import procedures if they wish to sell their products directly. Both of these mechanisms result in a negative impact on patients, who end up paying higher prices to cover unnecessary additional costs, in order to access high-quality, safe and effective medicines,” said a representative of the European Chamber of Commerce in Vietnam (EuroCham).
In essence, foreign investors, despite going through costly technology transfer, manufacturing, and registration procedures, are limited in their activities within the sector. This limitation makes investment in CMO, tolling, and pharmaceutical technology transfer for the production of various pharma products less appealing for foreign investors.
To incentivise this area in Vietnam, EuroCham has asked the government to expand the scope of the rights of FIEs. Specifically, enterprises which are the contract giver or contract acceptor performing the CMO or tech transfer should be allowed to perform activities related to distribution of the localised products in Vietnam by themselves. This is a-must-have-regulation to boost the local manufacturing of high-quality, affordable, safe, and effective medicines in Vietnam, EuroCham stated.
 | Outlook for pharmaceutical stocks in 2024 At a macro level in which there is still complexity, the pharmaceutical industry is expected to continue witnessing positive business results in 2024. |
 | Promoting Vietnamese pharmaceutical industry The Vietnamese pharmaceutical industry is seeing great advantages. |
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Related Contents
Latest News
More News
- Masan Consumer names new deputy CEO to drive foods and beverages growth (February 23, 2026 | 20:52)
- Myriad risks ahead, but ones Vietnam can confront (February 20, 2026 | 15:02)
- Vietnam making the leap into AI and semiconductors (February 20, 2026 | 09:37)
- Funding must be activated for semiconductor success (February 20, 2026 | 09:20)
- Resilience as new benchmark for smarter infrastructure (February 19, 2026 | 20:35)
- A golden time to shine within ASEAN (February 19, 2026 | 20:22)
- Vietnam’s pivotal year for advancing sustainability (February 19, 2026 | 08:44)
- Strengthening the core role of industry and trade (February 19, 2026 | 08:35)
- Future orientations for healthcare improvements (February 19, 2026 | 08:29)
- Infrastructure orientations suitable for a new chapter (February 19, 2026 | 08:15)

 Tag:
Tag:



















 Mobile Version
Mobile Version