Novavax starts Phase 1 clinical trial of COVID-19 vaccine
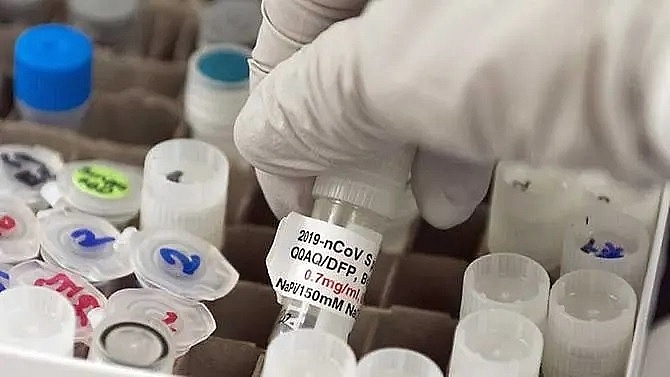 |
| A vial with a potential COVID-19 vaccine is seen at Novavax labs in Gaithersburg, Maryland. (ANDREW CABALLERO-REYNOLDS/AFP) |
The Maryland-based late-stage biotechnology company in April said it identified the candidate, NVX-CoV2373, with which it planned to use its Matrix-M adjuvant to enhance immune responses.
Adjuvants are mainly used to make vaccines induce a strong immune response, including through the greater production of antibodies, and provide longer-lasting protection against viral and bacterial infection.
Novavax said it expects preliminary immunogenicity and safety results from the trial in July.
The announcement comes as drugmakers pause clinical trials for other ailments and race to find an antidote for COVID-19, the illness caused by the novel coronavirus which has infected more than 5.3 million people worldwide and killed over 343,000.
Novavax said, upon successful completion of Phase 1, the Phase 2 portion of the trial will be conducted in several countries, including the United States.
The Phase 2 trial will assess immunity, safety and COVID-19 disease reduction in a broader age range, Novavax said.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
 Tag:
Tag:
Themes: COVID-19
- 67 million children missed out on vaccines because of Covid: UNICEF
- Vietnam records 305 COVID-19 cases on October 30
- 671 new COVID-19 cases recorded on October 1
- Vietnam logs additional 2,287 COVID-19 cases on Sept. 21
- People’s support decisive to vaccination coverage expansion: official
Related Contents
Latest News
More News
- Thailand asks Laos to waive visa fee at border checkpoints to boost tourism (October 21, 2024 | 17:23)
- Laos pledges to continue efforts to empower girls (October 21, 2024 | 17:17)
- Chinese electric vehicle maker to build plant in Indonesia (October 21, 2024 | 17:12)
- Vietnam Elevator Association introduces Elevator Safety Application to the world (October 18, 2024 | 09:00)
- A taste of the future - the go-to spot at the Worldchefs Congress & Expo 2024 (October 15, 2024 | 16:11)
- Jakarta to impose household waste levy (October 14, 2024 | 16:49)
- China, Laos plan to build connectivity development corridor with Thailand (October 14, 2024 | 16:19)
- Singapore keeps monetary policy unchanged (October 14, 2024 | 16:00)
- Indonesia aims to become key chain in global EV industry (October 14, 2024 | 15:59)
- RoK, Singapore to deepen AI, defence cooperation (October 09, 2024 | 16:18)























 Mobile Version
Mobile Version