Pfizer takes big steps with new vaccines
According to Pfizer, the stage one and two studies of the two-in-one vaccine showed positive results.
 |
| Senior production manager Ben Mees speaking to journalists about how the vaccines are made |
Thanks to this, Pfizer and its German partner BioNTech have moved a step closer to potentially receiving regulatory approval for a combination shot for COVID-19 and the flu.
The initial trials were conducted on healthy adults aged 18-64, who showed robust immune responses against influenza A, influenza B, and Sars-CoV-2 strains after getting the combination shot.
Dr. Annaliesa Anderson, senior vice-president and head of vaccine research and development at Pfizer said, “We are encouraged by these early results. This vaccine has the potential to lessen the impact of two respiratory diseases with a single injection and could simplify immunisation practices for providers, patients, and healthcare systems all over the world.”
Pfizer is also finalising the phase-three trials of its mRNA vaccine to protect adults from influenza.
At a meeting with journalists from VIR and those from other Southeast Asian nations on October 24 in Brussels, Dr Julia Spinardi, senior medical director for COVID-19 in emerging markets at Pfizer, said that the trial will evaluate the efficacy, safety, tolerability, and immunogenicity of the mRNA influenza vaccine.
 |
“The phase-three trial has been conducted only in the United States to take advantage of the flu season there. We had around 36,000 participants at more than 200 sites, and we are expecting to have results soon to present to the Food and Drug Administration,” she shared.
Dr. Mark Fletcher, Pfizer’s medical and scientific affairs lead for respiratory vaccines, added that every time there is a new technology, scientists try it on influenza.
“Now that we have the mRNA technology, which has been profoundly important for COVID-19, it has kicked off mRNA programmes and development for Pfizer,” he said.
“One other thing that is remarkable about the mRNA technology is it allows the concept of combination shots with other vaccines. You can actually target different viruses in a single injection with mRNA, and influenza is one of them,” added Fletcher.
 |
| Southeast Asian journalists visiting Pfizer’s manufacturing plant in Puurs, Belgium |
At the 41st Annual JP Morgan Healthcare Conference in January, Pfizer’s chairman and chief executive Albert Bourla said that the company could be ready to launch the two-in-one vaccine in 2024.
Now, people tend to hesitate to get booster doses, as COVID-19 is no longer considered a public health emergency. Experts, however, suggest individuals and governments should pay more attention to this.
Speaking at the Pfizer media meeting, Professor Tikki Pangestu, a visiting professor from the National University of Singapore's Yong Loo Lin School of Medicine said, “New variants can potentially increase the spread of the virus, even in populations with pre-existing immunity. We hope it does not happen, but the virus is unpredictable and can surprise us in ways we do not want, so vaccination should remain a public health priority.”
Dr. Leong Hoe Nam, from the Rophi Clinic in Singapore, added that in this endemic phase, the ground is still fertile for both infection and miscommunication, with misinformation stemming from social media causing hesitancy among some.
“Vaccine hesitancy continues to be a major concern. To fight this, we need to provide access to accurate, scientifically proven information to stave off misinformation on social media. We also need to debunk the lies and fake news,” he stated.
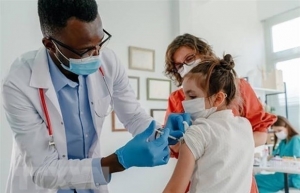 | Singapore authorises use of Pfizer’s COVID-19 vaccine on under-five children Singapore has recently approved Pfizer's Comirnaty COVID-19 vaccine for use on children aged six months to four years. |
 | Pfizer lifts 2022 forecast for Covid-19 vaccine sales as profits rise Pfizer reported higher quarterly profits Tuesday as it lifted its full-year forecast for coronavirus vaccine sales and predicted Covid-19 would yield billions more in revenues for the forseeable future. |
 | Pfizer seeks to boost presence in Vietnam A business mission led by Anil Argilla, president of Emerging Markets Asia at Pfizer, worked with Deputy Minister of Health Do Xuan Tuyen and the heads of other departments on May 25 to discuss business opportunities in Vietnam. |
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Themes: Healthcare Platform
- Opella and Long Chau join forces to enhance digestive and bone health
- Hanoi intensifies airport monitoring amid Nipah disease risks
- Cosmetics rules set for overhaul under draft decree
- Policy obstacles being addressed in drug licensing and renewal
- Sanofi, Long Chau Pharmacy relaunch medicine blister pack collection initiative
Related Contents
Latest News
More News
- A golden time to shine within ASEAN (February 19, 2026 | 20:22)
- Vietnam’s pivotal year for advancing sustainability (February 19, 2026 | 08:44)
- Strengthening the core role of industry and trade (February 19, 2026 | 08:35)
- Future orientations for healthcare improvements (February 19, 2026 | 08:29)
- Infrastructure orientations suitable for a new chapter (February 19, 2026 | 08:15)
- Innovation breakthroughs that can elevate the nation (February 19, 2026 | 08:08)
- ABB Robotics hosts SOMA Value Provider Conference in Vietnam (February 19, 2026 | 08:00)
- Entire financial sector steps firmly into a new spring (February 17, 2026 | 13:40)
- Digital security fundamental for better and faster decision-making (February 13, 2026 | 10:50)
- Aircraft makers urge out-the-box thinking (February 13, 2026 | 10:39)

 Tag:
Tag:




















 Mobile Version
Mobile Version