Sanofi and GSK to seek regulatory authorisation for COVID-19 vaccine
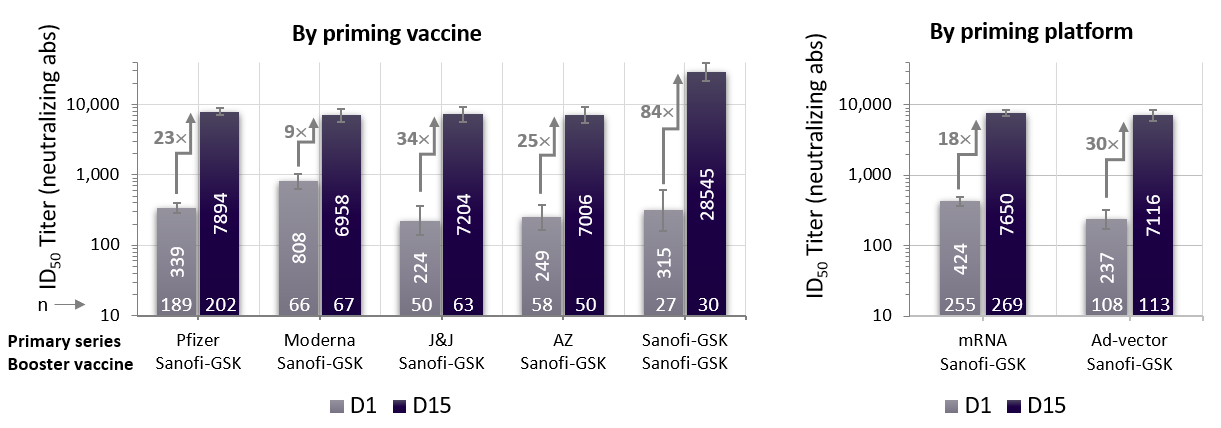 |
| Pre- vs post-booster neutralising antibody titers in 18-55-year old participants. Geometric Mean Titers (GMT) (95 per cent CI) |
The public health relevance of the refrigerator temperature-stable adjuvanted protein-based Sanofi-GSK vaccine is strongly supported by the induction of robust immune responses and a favourable safety profile in multiple settings.
In participants who had received a primary series of an already authorised mRNA or adenovirus vaccine, the Sanofi-GSK booster vaccine induced a significant increase in neutralising antibodies 18- to 30-fold across vaccine platforms and age groups.
When the Sanofi-GSK vaccine was used as a two-dose primary series followed by a booster dose, neutralising antibodies increased 84- to 153-fold compared to pre-boost levels.
Thomas Triomphe, executive vice president at Sanofi Vaccines said, “We’re very pleased with this data, which confirms our strong science and the benefits of our vaccine. The Sanofi-GSK vaccine demonstrates a universal ability to boost all platforms and across all ages. We also observed a robust efficacy of the vaccine as a primary series in today’s challenging epidemiological environment. No other global phase 3 efficacy study has been undertaken during this period with so many variants of concern, including Omicron, and the efficacy data is similar to the recent clinical data from authorised vaccines.”
Roger Connor, president of GSK Vaccines added, “The evolving epidemiology of COVID-19 demonstrates the need for a variety of vaccines. Our adjuvanted protein-based vaccine candidate uses a well-established approach that has been applied widely to prevent infection with other viruses including pandemic flu. We are confident that this vaccine can play an important role as we continue to address the pandemic and prepare for the post-pandemic period.”
When used as a two-dose primary series, the Sanofi-GSK vaccine delivered robust levels of neutralising antibodies, with GMTs reaching 3,711 units. For comparison, a panel of sera from volunteers in the same age range who received two doses of an already approved and highly effective mRNA vaccine displayed a GMT of 1,653 units, measured simultaneously in the same laboratory.
Data from the VAT08 efficacy study shows that two doses of Sanofi-GSK vaccine generated an efficacy of 57.9 per cent (95 per cent confidence interval CI, 26.5, 76.7) against any symptomatic COVID-19 disease in the seronegative population.
The Sanofi-GSK vaccine provided 100 per cent protection (0 vs 10 cases post-dose 1, 0 vs 4 cases post-dose 2) against severe disease and hospitalisations and 75 per cent (3 vs 11 cases) efficacy against moderate-to-severe disease in seronegative populations.
While sequencing is still in progress, early data indicates 77 per cent efficacy against any Delta variant-associated symptomatic COVID-19 disease, in line with expected vaccine effectiveness.
Across both studies, the Sanofi-GSK vaccine was well-tolerated in younger and older adults with no safety concerns.
The companies are in discussions with regulatory authorities and plan to submit the totality of the data generated with this vaccine candidate to support regulatory authorisations.
To evaluate the immunogenicity of the Sanofi-GSK vaccine as a booster, human immune sera samples were tested by Monogram Biosciences San Francisco using an FDA-approved standardised pseudovirus neutralisation test against the D614G prototype virus.
In the collaboration between the two companies, Sanofi provides its recombinant antigen and GSK contributes its pandemic adjuvant, both established vaccine platforms that have proven successful against influenza.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
 Tag:
Tag:
Themes: Healthcare Platform
- Opella and Long Chau join forces to enhance digestive and bone health
- Hanoi intensifies airport monitoring amid Nipah disease risks
- Cosmetics rules set for overhaul under draft decree
- Policy obstacles being addressed in drug licensing and renewal
- Sanofi, Long Chau Pharmacy relaunch medicine blister pack collection initiative
Related Contents
Latest News
More News
- Wages and Lunar New Year bonuses on the rise (February 09, 2026 | 17:47)
- Temporary relief for food imports as businesses urge overhaul of regulations (February 07, 2026 | 09:00)
- Opella and Long Chau join forces to enhance digestive and bone health (February 06, 2026 | 18:00)
- Vietnam-South Africa strategic partnership boosts business links (February 06, 2026 | 13:28)
- Sun PhuQuoc Airways secures AJW Group support for fleet operations (February 06, 2026 | 13:23)
- Pegasus Tech Ventures steps up Vietnam focus (February 05, 2026 | 17:25)
- The generics industry: unlocking new growth drivers (February 04, 2026 | 17:39)
- Vietnam ready to increase purchases of US goods (February 04, 2026 | 15:55)
- Steel industry faces challenges in 2026 (February 03, 2026 | 17:20)
- State corporations poised to drive 2026 growth (February 03, 2026 | 13:58)


























 Mobile Version
Mobile Version