Foreign pharma firms seek remedy for concerns
|
The forum will attract representatives from embassies in Vietnam, relevant ministries, and associations of foreign businesses, including members of Amcham.
This is an opportunity for them to discuss the impacts of these policies on their operations, as well as make suggestions to the Vietnamese government.
As planned, a circular guiding the implementation of Decree No.54/2017/ND-CP will be on the top of the agenda at the forum.
In May 2017, Vietnam issued Decree 54 guiding the implementation of the Law on Pharmaceuticals. The decree has caused great concern among foreign pharmaceutical companies, including members of AmCham and the European Chamber of Commerce in Vietnam (EuroCham).
According to EuroCham, the newly-issued decree finally allows importation, but also introduces a long list of restricted activities that render an importing foreign-invested enterprise (FIE) dysfunctional.
If a viable FIE was offered, the time needed to shift the operation model is estimated at around two years for most companies. Therefore, it is important to retain the current operations of representative offices (particularly drug information activities).
The decree also expands the definition of "distribution" activities, which could effectively drive FIEs providing warehousing and transportation services in Vietnam for over 20 years out of business.
As a result, it would be challenging for pharmaceutical multi-national corporations (MNCs) to identify partners that meet international quality and compliance requirements, and so supply continuity and product quality may be at risk.
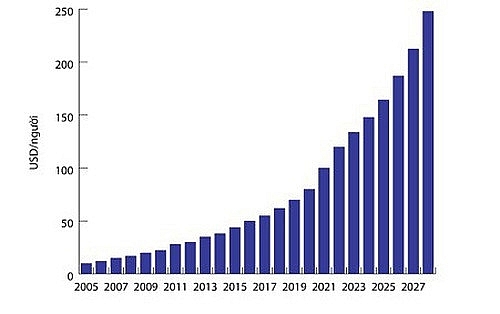
According to statistics from Business Monitor International (BMI), the Vietnamese pharmaceutical market is growing positively with revenue in 2017 estimated at $5.2 billion, up 10 per cent on-year. The market is forecast to continue to produce double-digit growth in the next five years.
What the stars mean:
★ Poor ★ ★ Promising ★★★ Good ★★★★ Very good ★★★★★ Exceptional
Related Contents
Latest News
More News
- Hermes joins Long Thanh cargo terminal development (February 04, 2026 | 15:59)
- SCG enhances production and distribution in Vietnam (February 04, 2026 | 08:00)
- UNIVACCO strengthens Asia expansion with Vietnam facility (February 03, 2026 | 08:00)
- Cai Mep Ha Port project wins approval with $1.95bn investment (February 02, 2026 | 16:17)
- Repositioning Vietnam in Asia’s manufacturing race (February 02, 2026 | 16:00)
- Manufacturing growth remains solid in early 2026 (February 02, 2026 | 15:28)
- Navigating venture capital trends across the continent (February 02, 2026 | 14:00)
- Motivations to achieve high growth (February 02, 2026 | 11:00)
- Capacity and regulations among British areas of expertise in IFCs (February 02, 2026 | 09:09)
- Transition underway in German investment across Vietnam (February 02, 2026 | 08:00)

 Tag:
Tag:






















 Mobile Version
Mobile Version