 |
On his first business trip to Vietnam, Russell Miller, vice president of Global Sales and Marketing at Enzene Biosciences, shared with VIR’s Ho Ha the company’s ambition to reshape the global biosimilars landscape through innovation and advanced manufacturing.
As one of India’s fastest-growing biopharmaceutical firms, Enzene is pursuing a model that combines affordability with world-class quality, an approach that aligns with Vietnam’s efforts to expand access to essential medicines and develop a more self-reliant healthcare industry.
He further discussed how Enzene’s next-generation manufacturing platforms are transforming the economics of biologics production, and why Vietnam, with its emerging market potential and growing R&D capacity, could become a strategic partner in the company’s global expansion.
Vice president Miller is a pharmaceutical expert with more than 20 years of experience driving growth and technology in the biopharmaceutical sector. His expertise spans the entire drug development process, from analytics and formulation to large-scale manufacturing.
In his current role at Enzene Biosciences, Miller focuses on developing strategic partnerships and expanding access to advanced biomanufacturing services worldwide. His leadership reflects Enzene’s broader vision of leveraging technology and innovation to deliver high-quality, affordable biologics to global markets.
|
 |
This is your first visit to Vietnam. After a busy week of meetings, what left the strongest impression on you?
What impressed me most was the opportunity to engage with senior officials from the Drug Administration of Vietnam (DAV) and to meet several dynamic Vietnamese pharmaceutical companies. These exchanges gave me a clear sense of the country’s determination to strengthen its healthcare system and accelerate the development of its pharmaceutical industry. I believe Enzene’s experience and biopharma solutions can play a meaningful role in improving both affordability and healthcare quality for Vietnamese patients.
I was also struck by the warmth and hospitality of the Vietnamese people. Everyone was incredibly welcoming and eager to share their culture through wonderful local cuisine. I particularly enjoyed Hanoi’s Pho and other traditional dishes. Today, we even tried a vegetarian restaurant, the creativity in using fresh ingredients was truly impressive.
 |
With more than two decades of experience in the pharmaceutical industry, could you share some of the key lessons from your career journey, and what ultimately inspired you to join Enzene Biosciences?
I began my career as a scientist in research and development, spending the first six years deeply involved in formulation and process innovation. Over time, I transitioned into commercial and strategic roles at companies such as Vectura, ANI Pharmaceuticals, Scotwork, and most notably Catalent Pharma Solutions, where I spent more than 16 years. These experiences gave me a comprehensive view of the pharmaceutical value chain – from R&D and clinical trials to biologics development and customer engagement – all guided by a commitment to improving patient outcomes.
What inspired me to join Enzene was the company’s clear vision for innovation and its strong technological foundation. Our CEO’s forward-looking approach, combined with Enzene’s proprietary, commercially proven technology platform, offers real potential to transform the way biologics are developed and manufactured. I saw in Enzene a rare opportunity to bring together scientific excellence, scalability, and affordability in a way that truly advances access to modern therapies.
The most important lesson I’ve learned in over 20 years is the power of effective planning. In pharma, navigating complex regulatory landscapes and managing risks requires meticulous preparation. Only with precise planning can we overcome global challenges and deliver meaningful benefits to patients worldwide.
 |
 |
In your view, what differentiates Enzene from competitors in the biosimilars and CDMO space? How does your technology optimise costs and enhance production efficiency?
That’s an excellent question. What truly sets Enzene apart is our clear vision and commitment to improving access to high-quality biologics. Our goal is to make advanced therapies more affordable, ensuring that patients, especially those in markets with limited healthcare access, can benefit from life-changing treatments.
Innovation lies at the core of our operations, guiding every stage from research and development to commercial manufacturing. Enzene’s technological foundation, combined with a culture of scientific creativity, allows us to maintain a pioneering edge while opening new opportunities for collaboration across the biopharmaceutical sector.
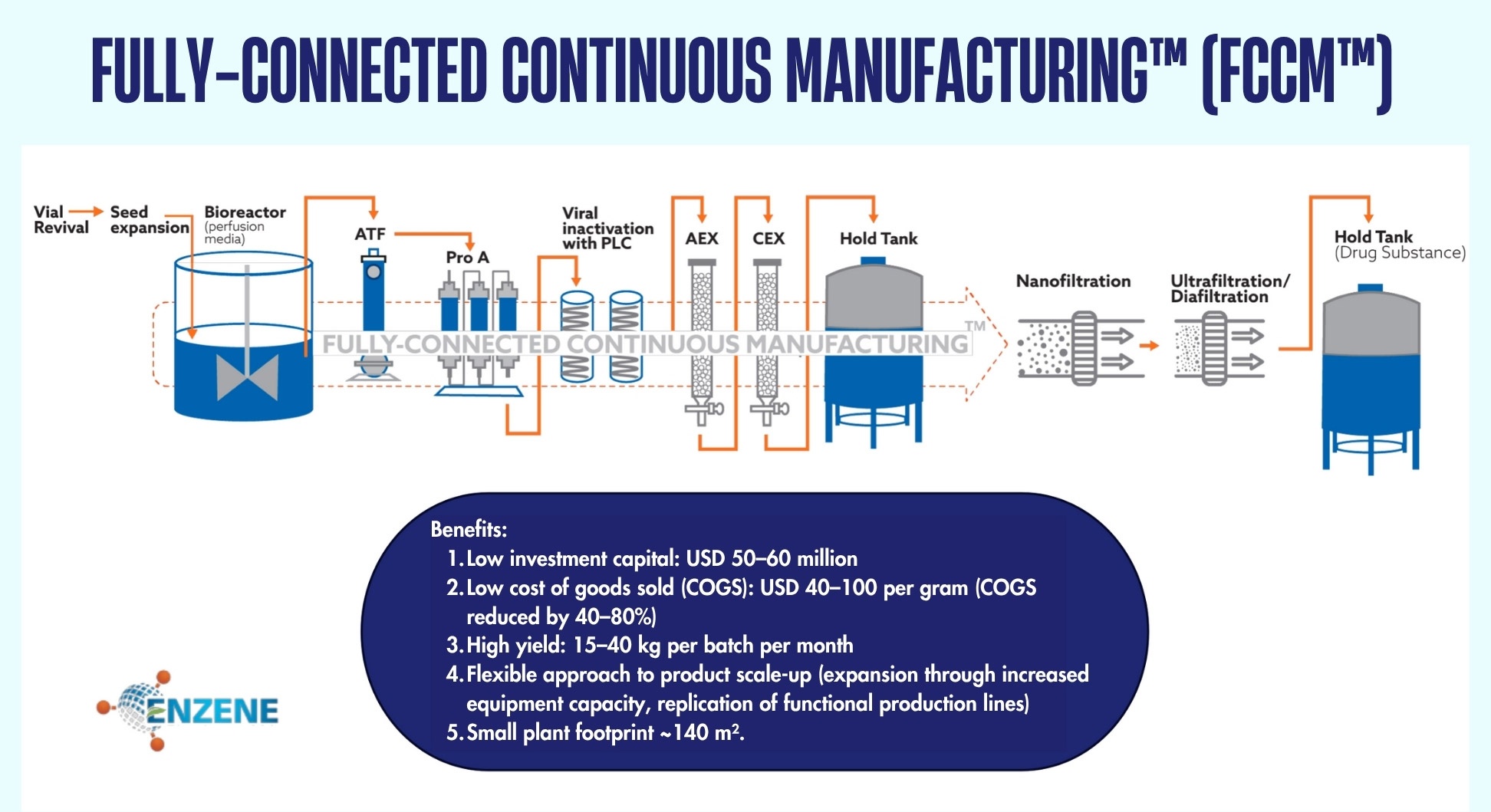
A central pillar of this innovation is our proprietary Fully-Connected Continuous Manufacturing (FCCM) platform, which seamlessly integrates all stages of biologics production – from upstream processing to downstream purification. Traditionally, biologics manufacturing requires multiple large bioreactors operating in sequence, often involving up to six massive tanks. This setup consumes substantial space, capital, and time, and introduces operational risks.
With FCCM, fresh media is continuously fed into cell cultures, linking reaction, separation, and purification into a single streamlined process. This approach maximises reactor utilisation, increases output by up to tenfold, significantly reduces costs, and minimises risk, representing a major step forward in biomanufacturing efficiency.
 |
For example, a 2,000-litre reactor previously required around 5,000 sq ft of manufacturing space. With FCCM, only 1,500 sq.ft is needed, saving 70 per cent in infrastructure costs. The system is also eco-friendly: higher productivity, lower cogs, consistent quality, and sustainable operations without the performance decline typical of batch processes.
With FCCM, we not only improve production efficiency but also accelerate patient access to vital biologics - with better quality and greater sustainability.
 |
Enzene has already developed seven biosimilars in India and is awaiting FDA and EMA approval for two others for global distribution. Could you share your ambitions for entering the global biopharma supply chain, and how long it might take to achieve that goal?
Over the past six years, Enzene has built a solid foundation for growth. In just three of those years, we successfully commercialised seven biosimilars, a milestone that reflects both the strength of our scientific platform and the dedication of our teams. Throughout this process, we have actively shared our technology and expertise with industry partners, fostering collaboration across multiple markets.
We have also made an important strategic transition, from being primarily a biosimilar developer to becoming a global contract development and manufacturing organisation (CDMO) with highly efficient and scalable processes.
 |
Our ambition is to establish Enzene as one of the world’s leading CDMOs for biologics within the next five years. To achieve this, we are expanding our manufacturing footprint in the United States to enhance our global reach, while advancing the next generation of our proprietary EnzeneX 2.0 platform.
Ultimately, our vision is to make a lasting contribution to global healthcare by providing high-quality, effective, and accessible biologic medicines to patients everywhere.
 |
 |
At the May workshop in Hanoi, Enzene’s presentation impressed Vietnamese pharmaceutical companies with its innovative approach to biosimilar production. What similarities between India and Vietnam make you confident about bringing this technology here?
Since joining Enzene two years ago, I have gained firsthand insight into the challenges India faced in making biologics widely accessible, while also studying Vietnam’s healthcare system.
Both countries share a common context: patients often bear the cost of advanced therapies out of pocket, as insurance coverage primarily extends to basic treatments. Income levels therefore have a direct impact on access to medicines. This is why we believe Enzene’s platform has the potential to deliver the same transformative impact in Vietnam that it achieved in India.
In India, for instance, Enzene supplies approximately 60 per cent of the market for Cetuximab, a biologic used to treat colorectal and head-and-neck cancers. Our technology has made these treatments affordable for the majority of patients. We see a similar opportunity in Vietnam to expand access to effective therapies, improving outcomes and delivering tangible benefits to patients across the country.
 |
In your opinion, which segment of the biopharma value chain should Vietnam prioritise first?
From my experience, Vietnam’s pharmaceutical companies are still in the early stages of developing expertise in biosimilars manufacturing. Establishing a strong foundation in this area is a crucial first step, as it equips local players with the capabilities needed to bring biologics to market and design effective treatment pathways.
For sustained growth, the sector will also need to strengthen knowledge in manufacturing processes, supply chain management, and leadership development. Building these capabilities will not only allow Vietnam to compete more effectively in the biosimilars market but also create opportunities to collaborate with, and learn from, established international CDMOs.
In short, biosimilars manufacturing represents the most practical starting point. It develops local expertise while laying the groundwork for future expansion into CDMO services and clinical trials.
 |
What capabilities must a Vietnamese company have to successfully receive technology transfer from Enzene, and what international lessons are most critical for building a competitive biopharma industry?
The first and most critical factor is a clear strategic vision: a well-defined starting point, long-term objectives, and a roadmap to achieve them. Our technology is a powerful enabler, but its impact depends on a company’s operational capacity and commitment – particularly for those seeking to compete in global markets.
In practice, three elements are essential. First, strategic vision to set goals and guide execution. Second, innovation to develop differentiated solutions. Third, a commitment to improving healthcare access, ensuring that patients and communities genuinely benefit. When these elements align, technology transfer can generate sustainable, high-impact value that differentiates the company in the marketplace.
Biopharma is inherently complex, especially for organisations building capabilities from the ground up. Companies can easily encounter technical or operational pitfalls. One of the most important international lessons is: don’t go it alone. Partnering with experienced teams not only helps avoid costly mistakes but also fosters creativity and accelerates learning. With the right collaboration, every new project has a far greater chance of success and long-term impact.
 |
And that’s precisely why you are in Vietnam - to connect, share experience, and support the sustainable growth of the country’s biopharma sector?
Exactly. From the outset, Enzene’s mission has been to expand access to therapies for patients who previously had limited opportunities. Operating as a CDMO, we prioritise quality and reliability to ensure that every dose reaches patients safely and on time.
Affordability underpins everything we do, and our technology enables this goal. We have been particularly impressed by the innovative drive of Vietnamese authorities, including the Ministry of Health, and by local companies striving to improve access and optimise therapies.
Our platform is certified under European GMP standards, with proven commercial manufacturing experience and a robust continuous production system. We believe that, whether through EnzeneX or other continuous manufacturing approaches, this technology will play a pivotal role in the future of biologics production in Vietnam.
 |
In global collaborations, what has been Enzene’s biggest regulatory or supply chain challenge, and how did the company overcome it?
Leading a global sales team, I’ve worked with partners across diverse markets, each with its own regulatory framework. The toughest challenge has consistently been ensuring that our products meet stringent standards in multiple jurisdictions, including Japan, the US, Canada, and the UK.
We addressed this by implementing a comprehensive quality system combined with a continuous manufacturing process that integrates every step from upstream to downstream, fully aligned with international regulations. Continuous production not only mitigates risk but also optimises efficiency and ensures the consistent quality of every dose.
Another key focus has been raising awareness about continuous cell-culture technology within the industry. The platform’s validation across multiple regulatory environments has enabled us to overcome barriers and deliver high-quality medicines worldwide.
For Enzene, continuous manufacturing represents not only a technological breakthrough, but also a strategic advantage in forming global partnerships.
 |
What common mistakes do latecomers make when entering the biopharma industry?
It’s a tough question. Many companies set strategies based on limited information or short-term goals. The biggest mistake is failing to invest adequately in technology and differentiation.
Some rely on outdated methods – for example, shortcut “fast path” approaches – without considering their current position or long-term objectives. They neglect to evaluate which technologies deliver sustainable value and fit their development strategies.
Another pitfall is choosing the wrong technology, which can set companies back significantly. We don’t claim Enzene’s technology is the only way forward, but continuous manufacturing undeniably reduces risk, increases productivity, and ensures quality. Companies in other markets face the same issues – they must carefully evaluate and adopt the right technology to remain competitive.
In summary, success in biopharma requires serious investment, smart technology choices, and clear differentiation from predecessors.
 |
 |
Could you explain the journey from producing chemical generics (bioequivalence) to biosimilars, and how Vietnamese patients will benefit once this technology is mastered?
If a company is already proficient in producing bioequivalents, the next step towards biosimilars is logical but by no means simple. Beyond existing clinical data and production processes, the company must build systems that demonstrate its ability to deliver reliable, consistent biologics.
The key lies in upgrading processes, adopting new technologies, and conducting thorough research to confirm clinical efficacy for patients. This ensures that biosimilars are not only up to standard but also competitive in the market.
For patients, this transition means far more than new drugs. Today, many therapies rely heavily on imports. Domestic biosimilar production will bring faster, more stable, and more affordable access to treatments. It’s also a knowledge revolution: Vietnamese biomedical teams mastering biosimilars will open doors to creating new therapies for a wide range of diseases.
Ultimately, successful implementation means Vietnam can not only serve domestic patients but also supply high-quality, globally competitive products - turning local capacity into global impact.
 |
In Vietnam, many Korean, Chinese, and Japanese firms are entering the market through mergers and acquisitions (M&As). Do you believe this will accelerate biopharma, and what are usually the main drivers behind such growth?
Vietnamese patients will benefit enormously from these activities. Biologics manufacturing is inherently complex, and foreign investors bring capital, technology, expertise, and infrastructure, which significantly advance the industry.
M&As are not just about financial investment. It enhances corporate capabilities, drives technology development, and prepares the industry for the future. Acquired or merged companies often boost R&D, improve facilities, and expand production capacity – all of which support long-term sector growth.
M&As offer Vietnam’s biopharma sector access to modern technology and abundant resources, preparing the country for next-generation biologics while delivering tangible benefits to patients.
 |
 |
During this trip, what results do you expect from working with the Ministry of Health and local companies? How does Enzene envision long-term collaboration in Vietnam?
This trip gave us a valuable opportunity to meet regulators, experts, and businesses, and to understand the vision and development priorities of Vietnam’s biopharma industry.
These discussions help us align our market approach with real-world needs, regulatory frameworks, and expectations for safe, high-quality, and affordable biologics. The openness and collaborative spirit of the Ministry of Health and the DAV have been invaluable, bringing us closer to our mission of improving patient access in Vietnam.
As a CDMO, we want to accompany Vietnamese companies at every step of product development - from leveraging existing portfolios to creating new therapies – while providing technology, expertise, and resources to help them succeed.
We came to Vietnam with a passion for driving innovation, building sustainable value, and expanding patient access, while also learning from the dedicated local teams. Our long-term goal is to help Vietnam’s biopharma sector grow stronger, enhance healthcare quality, and prepare for opportunities to enter the global market.
 |
Enzene Biosciences, a subsidiary of Alkem Laboratories – one of India’s top five pharmaceutical groups – is a biotechnology company specialising in biosimilars, novel biologics, and CDMO/CMO services. As a pioneer in next-generation biologics technologies, Enzene is disrupting the existing biologics manufacturing paradigm with its patented EnzeneX technology, which was the FCCM platform validated for commercial biologics supply.
Enzene partners with innovators and biosimilar developers to deliver accelerated time-to-market, increased production yields, and cost reductions across a broad range of biologic modalities. Committed to pushing the boundaries of biologics manufacturing innovation, Enzene is striving to reduce monoclonal antibody production costs to below $40 per gram by 2025.
With three manufacturing and research facilities in India and one in the United States, Enzene has developed seven biosimilars for the Indian market: Bevacizumab, Cetuximab, Denosumab, Adalimumab, Ranibizumab, Romiplostim, and Teriparatide. An eighth product, Pertuzumab, is expected to launch in October. The company is also working with the US Food and Drug Administration and the European Medicines Agency for the approval of two biosimilars for global distribution.
CONTENT & DESIGN: HO HA - PHOTOGRAPHY: CHI CUONG
 |